Health
Samsung Biologics completes full acquisition of Samsung Bioepis
* Samsung Biologics has now fully acquired Samsung Bioepis, as a wholly owned subsidiary * First payment completed funded through paid-in capital * Accelerated growth expected in biosimilars and future novel therapeutics R&D INCHEON,South Korea, April 20, 2022 /PRNewswire/ -- Samsung Biologi...
FDA granted CMG901 Fast Track Designation for unresectable or metastatic gastric and gastroesophageal junction cancer which have relapsed and/or are refractory to approved therapies
CHENGDU, China, April 19, 2022 /PRNewswire/ -- Keymed Biosciences (2162.HK) announced that the U.S. Food and Drug Administration (FDA) granted CMG901 Fast Track Designation as monotherapy for the treatment of unresectable or metastatic gastric and gastroesophageal junction cancer which have relap...
ImmVira enters clinical development in combination therapy of MVR-T3011 IT and MEK inhibitor in the U.S.
SHENZHEN, China, April 19, 2022 /PRNewswire/ -- ImmVira announced that, company has reached a cooperation agreement with Roche to establish clinical research partnership recently, to conduct clinicalstudies in the U.S. on the combination therapy of ImmVira's MVR-T3011 IT and Roche's MEK inhibitor...
Paving the Last Mile, Fosun Foundation Delivers Essentials to Hundreds of Communities in Shanghai
HONG KONG, April 19, 2022 /PRNewswire/ -- Since the latest COVID-19 outbreak which started in March inShanghai, China, Fosun Foundation has been promptly responding to the needs from the city's universities, hospitals, and communities. OnApril 1, Fosun Foundation initiated the "Community Support ...
Mediscience Espoir Inc. Looking for OEM Customers, Technical Business Partners and Sales Agents for Oxygen Clathrate Hydrates and Oxygen Solutions
KAWASAKI, Japan, April 19, 2022 /PRNewswire/ -- Mediscience Espoir Inc. announced onApril 19 that it is looking for U.S. partners for oxygen clathrate hydrates and oxygen solutions based on its proprietary technology. The company obtained "Substance Patent" in the United States (US 10,913,037 B2)...
Bridge Biotherapeutics Enters into an Exclusive License Agreement with Shaperon to Strengthen Its IPF Franchise
* The licensing deal includes an upfront payment of USD 1.63 million, and may reach up toUSD 24.4 million in milestone payments and royalties * By strengthening the company's fibrotic disease development pipeline, Bridge will enhance its strategic focus on the development of comprehensive tre...
GeneQuantum and AIMEDBIO Collaborate on a First-in-Class Antibody -Drug-Conjugate
SUZHOU, China, April 18, 2022 /PRNewswire/ -- GeneQuantum Healthcare (Suzhou) Co., Ltd., a company specializing in site-specific bioconjugation for next generation biotherapeutics, has signed an agreement with a Korean biotech company, Aimed Bio Inc., to co-develop a First-in-Class therapeutic an...
A Brainwave Technology from Hyundai Mobis Proven to Reduce Drowsiness and Inattentive Driving by Up to 1/3
* M.Brain, the world's first brainwave technology developed last year, proven to be effective in a pilot application. * Cuts down drowsiness after meals by 30% and on highways by 20% max. Also, reduces time taken to recover attention by three times. * Hyundai Mobis secures competitiveness...
WuXi Biologics Receives Bioprocessing Excellence Award from IMAPAC
SINGAPORE, April 14, 2022 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a global Contract Research, Development and Manufacturing Organization (CRDMO) service company, today announced that the company received the award for Bioprocessing Excellence in Viral Clearance and Safety at the A...
Asieris Pharmaceuticals Announces 2021 Annual Report: Steady Progress in Core Product Pipeline and Progressive Implementation of the Integrated Diagnosis and Treatment Commercialization Strategy
SHANGHAI, April 14, 2022 /PRNewswire/ -- Asieris Pharmaceuticals (688176.SH), a global innovative pharmaceutical company specializing in the treatment of genitourinary cancers and related major diseases, announced its 2021 annual report today. The company is currently making steady progress in mu...
Gracell Biotechnologies' Abstract on GC012F, a BCMA/CD19 Dual-targeting CAR-T Cell Therapy, Accepted for Presentation at 2022 ASCO Annual Meeting
PALO ALTO, Calif. and SUZHOU, China, April 14, 2022 /PRNewswire/ -- Gracell Biotechnologies Inc. ("Gracell" or the "Company", NASDAQ: GRCL), a global clinical-stage biopharmaceutical company dedicated to developing highly efficacious and affordable cell therapies for the treatment of cancer, toda...
China Medical System (0867.HK): the Leader in Commercialization Embraces Great Industry Opportunities as Biotech Companies are Eager to Monetize Their Innovative Products
HONG KONG, April 14, 2022 /PRNewswire/ -- Recently, China Medical System ("CMS", 0867.HK) announced its annual results. After recording continuous performance growth in the difficult time of the pandemic in 2020, it once again achieved a new record high, with revenue ofRMB 8,337 million, up 20.0%...
Fosun Diagnostics Receives China NMPA Approval for Its Independently Developed Novel Coronavirus Antigen Detection Kit Amidst of The National Efforts in Pandemic Prevention and Control
SHANGHAI, April 14, 2022 /PRNewswire/ -- April 13, 2022, Shanghai Fosun Pharmaceutical (Group) Co., Ltd. ("Fosun Pharma" or "the Group"; Stock Code: 600196.SH, 02196.HK) announced that the Novel Coronavirus (2019-nCoV) Antigen Detection Kit (Colloidal Gold) self-developed by Fosun Diagnostics Co....
Akeso Publishes Preclinical Results of PD-1/LAG-3 Bi-specific Antibody(AK129)at the American Association for Cancer Research (AACR) 2022 Annual Meeting
HONG KONG, April 14, 2022 /PRNewswire/ -- Akeso, Inc. (9926.HK) ( "Akeso" ), a China-based biopharmaceutical company focusing on the development and commercialization of innovative therapeutic antibodies for Oncology & Immunology, announced the publication of preclinical results of its PD-1/LAG-3...
Akeso Publishes Preclinical Results of PD-1/CD73 Bi-specific Antibody(AK131)at the American Association for Cancer Research (AACR) 2022 Annual Meeting
HONG KONG, April 14, 2022 /PRNewswire/ -- Akeso, Inc. (9926.HK) ( "Akeso" ), a China-based biopharmaceutical company focusing on the development and commercialization of innovative therapeutic antibodies for Oncology & Immunology, announced the publication of preclinical results of its PD-1/CD73 ...
Akeso Publishes Preclinical Results of TIGIT monoclonal antibody(AK127)at the American Association for Cancer Research (AACR) 2022 Annual Meeting
HONG KONG, April 14, 2022 /PRNewswire/ -- Akeso, Inc. (9926.HK) ( "Akeso" ), a China-based biopharmaceutical company focusing on the development and commercialization of innovative therapeutic antibodies for Oncology & Immunology, announced the publication of preclinical results of its anti-TIGIT...
Toyobo launches major marketing initiative for Nerbridge(TM) in the U.S., setting sights on the global nerve conduit market
OSAKA, Japan, April 13, 2022 /PRNewswire/ -- Toyobo Co., Ltd. has launched a major U.S. marketing initiative for NerbridgeTM ("the product", hereafter), a conduit for peripheral nerve regeneration. On January 11, 2022, Toyobo kicked off the sales promotion campaign at a conference jointly held i...
Harbour BioMed Appoints Dr. Humphrey Gardner as Chief Medical Officer
CAMBRIDGE, Mass., SUZHOU, China and ROTTERDAM, Netherlands, April 13, 2022 /PRNewswire/ -- Harbour BioMed ("HBM" or the "Company"; HKEX: 02142), today announced the appointment of Dr.Humphrey Gardner as Chief Medical Officer (CMO). Dr. Gardner will be responsible for leading the global product d...
AACR 2022 | Ascentage Pharma Presents Latest Results from Six Preclinical Studies at AACR Annual Meeting 2022
SUZHOU, China and ROCKVILLE, Md., April 13, 2022 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that it has presented the latest results from six...
InxMed FAK Inhibitor IN10018 Received Breakthrough Therapy Designation by the China National Medical Products Administration for Platinum-Resistant Ovarian Cancer
NANJING, China, April 13, 2022 /PRNewswire/ -- InxMed Co., Ltd. announced that IN0018, its focal adhesion kinase (FAK) inhibitor, had been granted with Breakthrough Therapy Designation by the China National Medical Products Administration. This designation is based on the results of a Phase Ib...
Week's Top Stories
Most Reposted
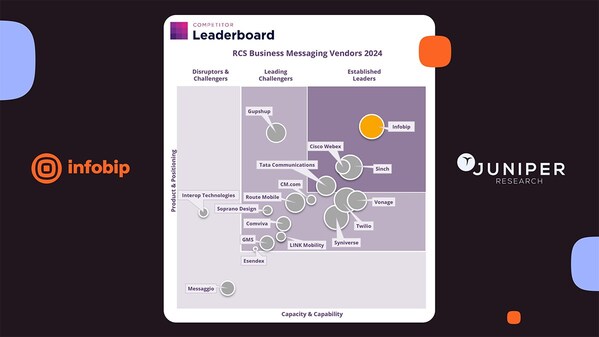
Infobip recognized as RCS Business Messaging Leader by Juniper Research
[Picked up by 331 media titles]
2024-11-05 18:06Mastercard goes OTP-free in APAC for faster, safer online transactions
[Picked up by 326 media titles]
2024-11-06 09:00Brankas Launches Integrated APAC Open Banking Compliance Solution with ADVANCE.AI's eKYC Solution
[Picked up by 319 media titles]
2024-11-07 09:00Mastercard launches Pay Local, enabling Asia's digital wallet providers to process card payments from more than 2 billion Mastercard cardholders
[Picked up by 317 media titles]
2024-11-05 09:00XtalPi and Sinar Mas Multiartha Launch Strategic Partnership to Revolutionize AI Across Asia-Pacific
[Picked up by 277 media titles]
2024-11-07 11:34