Biotechnology
HanAll Biopharma Announces Initiation of Phase III Randomized, Double-Masked Vehicle Controlled VELOS-4 Trial Evaluating Tanfanercept for Treatment of Dry Eye Disease
* HanAll has initiated a Phase III VELOS-4 study to evaluate the efficacy and safety of tanfanercept in dry eye based on the findings from the previous Phase III VELOS-3 study. * Tanfanercept demonstrated statistically significant improvement on the secondary outcome measure, Schirmer testing...
Integrated cancer biotech Infinitopes secures £12.8m seed financing to enhance its Precision Immunomics™ antigen discovery technologies to target five more cancers
Funding also supports the start of phase I/IIa study for lead vaccine, ITOP1 OXFORD, England, May 2, 2024 /PRNewswire/ -- Infinitopes Precision Immunomics, an integrated cancer biotech combining world leading platforms in precision antigen discovery with vaccine vectors capable of durably stimula...
Shineco Develops Revolutionary New Product with Varied Applications to Positively Impact the Health Care Industry
BEIJING, May 1, 2024 /PRNewswire/ -- Shineco, Inc. ("Shineco" or the "Company"; NASDAQ: SISI), a provider of technologically advanced healthcare products and services, announced today that its subsidiaries Fuzhou Medashan Biotechnology Co., Ltd. and Kaifeng Yixi Biotechnology Co., Ltd. have innov...
Eluminex Biosciences Announces FDA Acceptance of Investigational New Drug (IND) Application for EB-105 - A Novel Trispecific Fusion Antibody for Diabetic Macular Edema (DME) - and Upcoming Scientific Presentations
SAN FRANCISCO and SUZHOU, China, April 30, 2024 /PRNewswire/ -- Eluminex Biosciences (Eluminex), a privately-held biotechnology company focused on the development of advanced protein therapeutics for vision-threatening diseases and dermal facial aesthetics announced the acceptance of their EB-105...
National Medical Products Administration (NMPA) Approves Chipscreen Bioscience's Chidamide (Epidaza) combined with R-CHOP for the treatment of diffuse large B-cell lymphoma
SHENZHEN, China, April 30, 2024 /PRNewswire/ -- Shenzhen Chipscreen Biosciences Co., Ltd. (Chipscreen Biosciences, Stock Symbol: 688321.SH) announced that the company's lead innovative product Chidamide (Epidaza®) , an oral subtype-selective histone deacetylase (HDAC) inhibitor, combined with R-C...
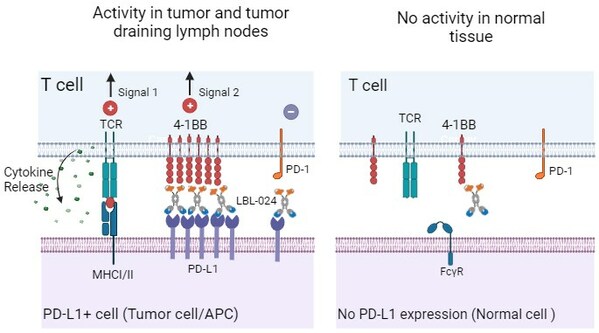
A Potential First-In-Class Drug: CDE Approved Single-Arm Pivotal Clinical Study of LBL-024, An Anti-PD-L1/4-1BB Bispecific Antibody Developed by Leads Biolabs
NANJING, China, April 30, 2024 /PRNewswire/ -- Nanjing Leads Biolabs Co., Ltd. (hereinafter referred to as "Leads Biolabs") announced that LBL-024, an anti-PD-L1/4-1BB bispecific antibody independently developed by Leads Biolabs with global intellectual property rights has received approval to co...
HanAll Biopharma Reports Q1 2024 Financial Results and Provides Business Update
* Delivered solid performance to start 2024, with record-breaking first quarter revenue of34.1 billion KRW. Strong sales momentum continued from key products, funding investments in ongoing R&D programs. * Phase 3 VELOS-4 study of tanfanercept in dry eye disease expected to be initiated in th...
WuXi Biologics Releases 2023 ESG Report Demonstrating Strong Sustainability Commitment
* The Company demonstrated a deep commitment to achieving ESG success in partnership with global clients, creating long-term value for all stakeholders. * The Company made remarkable progress in tackling climate change, achieving a 29% intensity reduction of Scope 1 and Scope 2 greenhouse gas ...
CARsgen Submitted Responses to FDA Observations
SHANGHAI, April 29, 2024 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces that responses regarding the status of the Corrective and Preventive...
Technoderma Medicines Advances TDM-180935 Atopic Dermatitis Clinical Program with Phase 2 Trial
CHENGDU, China, April 29, 2024 /PRNewswire/ -- Technoderma Medicines, Inc. ("the Company"), a clinical stage biopharmaceutical company, is pleased to report the Company has begun dosing patients in its Phase 2a clinical trial (NCT06363461) of topical TDM-180935 ointment. This clinical trial in t...
YS Biopharma Receives Additional 180 Day Extension by Nasdaq to Regain Compliance with Minimum Bid Price Rule
GAITHERSBURG, Md., April 29, 2024 /PRNewswire/ -- YS Biopharma Co., Ltd. (Nasdaq: YS) ("YS Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for infectious dise...
WuXi AppTec Achieved First Quarter 2024 Target Despite External Challenges, Maintaining Stable Operations
* Revenue Reached RMB7,982 Million; Excluding COVID-19 Commercial Projects, Down 1.8% * Net Profit Attributable to the Owners of the Company Reached RMB1,942 Million; Diluted Earnings per Share (EPS) Reached RMB0.66 * Adjusted Non-IFRS Net Profit Attributable to the Owners of the Company Re...
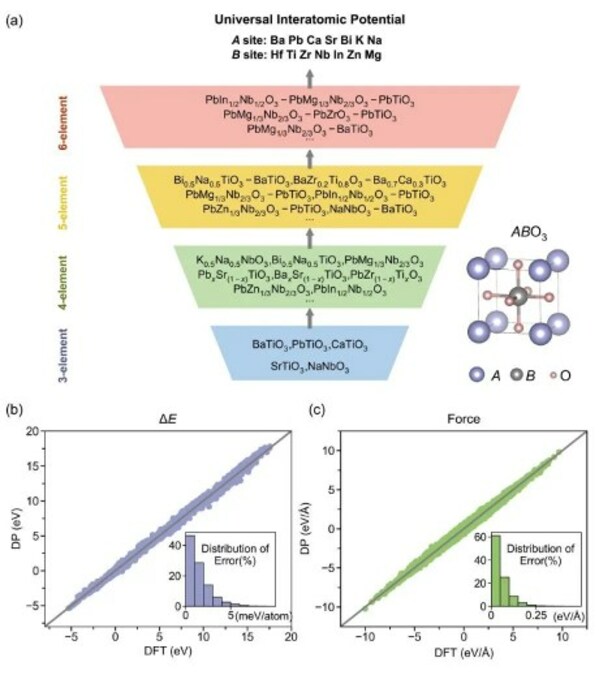
DP Technology DevDay 2024 Showcases Large Science Models and Announces Open Science Initiative
BEIJING, April 29, 2024 /PRNewswire/ -- In recent years, the rapid development of artificial intelligence has introduced new possibilities across numerous scientific disciplines. As an AI for Science pioneer, DP Technology is continually collaborating with partners to explore the transformative i...
Ractigen Therapeutics Announces FDA Approval for RAG-01, a First-in-Class saRNA Therapy for BCG-Unresponsive NMIBC
SUZHOU, China, April 26, 2024 /PRNewswire/ -- Ractigen Therapeutics, a leader in the development of small activating RNA (saRNA) therapeutics, announced today that the U.S. Food and Drug Administration (FDA) has approved the company's Investigational New Drug (IND) application for RAG-01, a grou...
Novel T-cell engager, CDH17 X CD3 cabotamig (ARB202) continues to explore dosing in patients with advanced gastrointestinal cancers
* The second DMC review recommends the continuation of dose escalation in the clinical trial as per protocol. * The interim safety assessment was based on the review of safety data from 18 metastatic gastrointestinal cancer patients, including patients that have tolerated multiple doses of ca...
Fosun Pharma's Self-developed Artemisinin Medicines Inject New Impetus to Malaria Prevention and Treatment in Africa
SHANGHAI, April 26, 2024 /PRNewswire/ -- World Malaria Day is marked each year onApril 25. World Health Organization (WHO) gave as the theme for World Malaria Day 2024Accelerating the fight against malaria for a more equitable world. WHO stated that malaria not only continues to directly endanger...
Caliway Announced CBL-514 Phase 2 Study for Cellulite Treatment Met All Primary and Secondary Endpoints
* CBL-514 is the first product to treat cellulite at the raised areas. * Currently, there is no effective and safe cellulite treatment on the market. The global market for cellulite treatment is estimated to expand to $7.37 billion in 2034. * CBL-0201EFP Phase 2 study demonstrated CBL-514 sta...
2024 ASCO | Mabwell to Present Clinical Data of 9MW2821 in Multiple Advanced Solid Tumor
SHANGHAI, April 25, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with an entire industry chain, announced that the Phase I/II clinical study results of the novel Nectin-4-targeting ADC 9MW2821 for multiple advanced solid tumors will be presented in an ...
Hyundai Bioscience's antiviral for Dengue Fever to enter clinical trials in Brazil
* "Aiming to offer a Dengue antiviral treatment course in $100 price range" * Hyundai Bioscience decides to conduct dengue clinical trials in Brazil, which has the largest number of Dengue patients and offers a fast-track approval SEOUL, South Korea, April 25, 2024 /PRNewswire/ -- Hyundai Biosc...
Hyundai Bioscience Announces Clinical Development Plan for Niclosamide-based Metabolic Anticancer Drug Targeting P53 Mutation Cancer
* The only solution for intractable cancer caused by mutations in the p53 gene * Planning clinical trials targeting cancer patients with no available treatment due to p53 gene mutations SEOUL, South Korea, April 25, 2024 /PRNewswire/ -- Hyundai Bioscience announced onApril 25th that its clinica...
Week's Top Stories
Most Reposted
LINKDOOD Breaks Language Barriers, Ushering a New Era for Cross-Border Romance
[Picked up by 326 media titles]
2024-04-29 06:00Multiple achievements made in China-Hungary BRI conference
[Picked up by 310 media titles]
2024-05-03 06:59Dow showcases circular and innovative materials science solutions and industry collaborations at Chinaplas 2024
[Picked up by 293 media titles]
2024-04-30 10:11Xinhua president, Hungarian economy minister vow to bolster media cooperation
[Picked up by 281 media titles]
2024-05-03 06:25Puyuan Fashion Resort 2024: A Grand Unveiling of Global Trends and Local Heritage
[Picked up by 269 media titles]
2024-04-28 09:39