Biotechnology
Ractigen Announces First Patient Dosed in the Phase I Clinical Trial of RAG-01 for NMIBC
JIANGSU, China, April 3, 2024 /PRNewswire/ -- Ractigen Therapeutics, a pioneer in small activating RNA (saRNA) therapeutics, achieved a major milestone today with the first patient dosed in its First-in-human phase I clinical trial for RAG-01 conducted in collaboration with GenesisCare,Australia'...
Ascletis Announces Strategic Decisions on FXR agonist ASC42
HANGZHOU, China and SHAOXING, China, April 3, 2024 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX:1672, "Ascletis") announces today the strategic decisions on farnesoid X receptor (FXR) agonist ASC42. After thorough analysis of the Phase II trial data of ASC42 for primary biliary cholangitis (PBC) (...
Nagase Viita Receives Highest "Platinum" Rating for Sustainability from EcoVadis
OKAYAMA, Japan, April 3, 2024 /PRNewswire/ -- Nagase Viita Co., Ltd., a NAGASE Group member headquartered in Okayama,Japan, has received the highest "Platinum" rating in a sustainability survey conducted by EcoVadis SAS ofFrance , one of the world's most trusted providers of business sustainabilit...
I-Mab Announces Closing of the Divestiture of Business Operations in China
ROCKVILLE, Md., April 2, 2024 /PRNewswire/ -- I-Mab (the "Company") (NASDAQ: IMAB) a U.S.-based, global biotech company, exclusively focused on the development and potential commercialization of highly differentiated immunotherapies for the treatment of cancer, today announced that all condition...
GC Cell to Present Multiple Posters at the American Association for Cancer Research (AACR) Annual Meeting 2024
YONGIN, South Korea, April 2, 2024 /PRNewswire/ -- GC Cell
First Patient Dosed in Clinical Trial of YOLT-101 for the Treatment of FH
SHANGHAI, April 2, 2024 /PRNewswire/ -- YolTech Therapeutics announced that the first patient has been dosed with YOLT-101, the company'sin vivo genome editing candidate being developed as a single dose, potentially curative therapy for Familial Hypercholesterolemia(FH), marking the commencement ...
Origin Agritech Provides First Half Revenue Forecast and Updates Advancements in Hybrids and GMO Development
Estimated Revenue Growth of 30-40% in the First Half of FY 2024 Two New Hybrids Completed National Trials and Four GMO Corn Hybrids Currently in National Trials BEIJING, April 2, 2024 /PRNewswire/ -- Origin Agritech Ltd. (NASDAQ: SEED) (the "Company" or "Origin"), a leading Chinese agricultural ...
Lunit to Showcase 7 Studies at AACR 2024: Unveiling AI Innovations in HER2 Expression-Mutation Analysis and CNTN4 Biomarker Identification
- Lunit's AACR 2024 presentations to spotlight HER2 mutation insights and CNTN4's role in immunotherapy success, pioneering next-gen personalized treatment approaches, supported by the AI-powered Lunit SCOPE suite SEOUL, South Korea, April 1, 2024 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading...
Aculys Pharma Announces Appointment of Hidemasa Tanigaki as New CEO
~Hidemasa's appointment bolsters Aculys' position as a leader in the field of CNS inJapan and Asia~ TOKYO, April 1, 2024 /PRNewswire/ -- Aculys Pharma, Inc. ("Aculys"), a clinical-stage biopharmaceutical company focused on commercializing innovative treatments for neurological conditions, today ...
LISCure Biosciences receives U.S. FDA Fast Track designation for LB-P8 for the treatment of primary sclerosing cholangitis (PSC)
* Phase 2 study is underway and LB-P8 is the only live biotherapeutic product currently reported to be in clinical development for the treatment of PSC * FDA's Fast Track designation for LB-P8 underlines the urgent need for treatment options to fulfill the unmet medical needs of people affecte...
73% CNS ORR! FDA Granted ODD to Utidelone Injectable (UTD1) from Biostar Pharma for the Treatment of Breast Cancer Brain Metastasis
SAN FRANCISCO, March 29, 2024 /PRNewswire/ -- Biostar Pharma, Inc., the U.S. subsidiary of Beijing Biostar Pharmaceuticals Co., Ltd. which is a synthetic biology driven biopharma company focusing on the development and commercialization of innovative oncology drugs, announced today that their cor...
Alebund's Innovative Investigational Drug AP303 Receives FDA Orphan Drug Designation (ODD) for the Treatment of Autosomal Dominant Polycystic Kidney Disease (ADPKD)
SHANGHAI, March 29, 2024 /PRNewswire/ -- Alebund Pharmaceuticals
IASO Bio Announces NMPA's IND Approval for Equecabtagene Autoleucel in Second- and Third-Line Treatment of Multiple Myeloma
SHANGHAI and NANJING, China and SAN JOSE, Calif., March 29, 2024 /PRNewswire/ -- IASO Bio, a biopharmaceutical company engaged in discovering, developing, manufacturing and marketing innovative cell therapies and antibody products, today announced thatChina National Medical Products Administratio...
Jacobio Pharma Announces 2023 Annual Results
BEIJING, SHANGHAI and BOSTON, March 28, 2024 Jacobio/PRNewswire/ -- Pharma (1167.HK), a clinical-stage oncology company drugging the undruggable targets, today announced its 2023 annual results. The revenue wasRMB63.5 million, the R&D investment wasRMB372 million, the cash and cash equivalent at...
Bridge Biotherapeutics Launches a Research Collaboration with Emory University School of Medicine to Explore Combination Therapy of BBT-877 for KRAS/P53 Mutant NSCLC Patients Resistant to Anti-PD-1 Blockade
SEONGNAM, South Korea and ATLANTA, March 28, 2024 /PRNewswire/ --
Bridge Biotherapeutics (KQ288330)
Harbour BioMed Reports Full Year 2023 Financial Results
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, March 28, 2024 /PRNewswire/ -- Harbour BioMed ("HBM" or the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development and commercialization of novel antibody therapeutics focusing on immune-oncol...
GenFleet and BeiGene Enter into Trial Collaboration for a Potentially First-in-class Combination Therapy to Initiate Phase Ib/II Study of GFH009 (CDK9 inhibitor) and BRUKINSA® (zanubrutinib) Treating Diffuse Large B Cell Lymphoma
SHANGHAI, March 28, 2024 /PRNewswire/ -- GenFleet Therapeutics, a clinical-stage biotechnology company focusing on cutting-edge therapies in oncology and immunology, today announced it has entered into a clinical trial collaboration and supply agreement with BeiGene Switzerland GmbH to start a c...
Tigermed Reports Full Year 2023 Results
HANGZHOU, China, March 28, 2024 /PRNewswire/ -- Hangzhou Tigermed Consulting Co., Ltd. ("Tigermed" or the "company") (Stock code: 300347.SZ / 3347.HK), a leading global provider of integrated research and development solutions for biopharmaceutical and medical device industry, announced its annua...
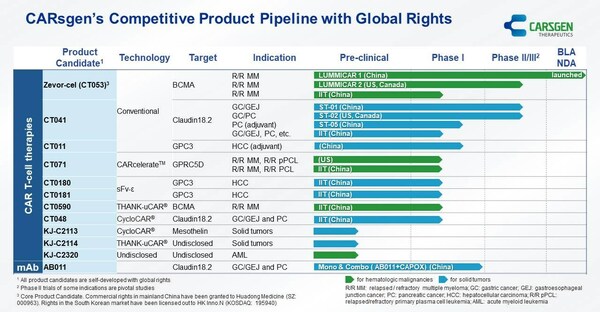
CARsgen Announced 2023 Annual Results
SHANGHAI, March 27, 2024 /PRNewswire/ -- March 27, 2024, CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announced its 2023 Annual Results. Business Highlights * Z...
Sanyou Bio: Sanyou's Intelligence-Enabled Innovative Biologics R&D Platform Leads in the New Era of Biological Drug Development
SHANGHAI, March 27, 2024 /PRNewswire/ -- Dr. Guojun Lang, founder and CEO of Sanyou Biopharmaceuticals provides industry insights: The new era sees the transition towards digitalization and intelligence. Following this trend, we have been thinking and planning the transformative path of digitali...
Week's Top Stories
Most Reposted
LINKDOOD Breaks Language Barriers, Ushering a New Era for Cross-Border Romance
[Picked up by 326 media titles]
2024-04-29 06:00Dow showcases circular and innovative materials science solutions and industry collaborations at Chinaplas 2024
[Picked up by 291 media titles]
2024-04-30 10:11Puyuan Fashion Resort 2024: A Grand Unveiling of Global Trends and Local Heritage
[Picked up by 269 media titles]
2024-04-28 09:39Chinese new energy industry contributes to global green, low-carbon transition
[Picked up by 249 media titles]
2024-04-30 15:13New International Land-Sea Trade Corridor achieves rapid development
[Picked up by 246 media titles]
2024-04-28 10:24