Pharmaceuticals
AffaMed Therapeutics Announces Completion of Patient Enrollment in the Real-World Study in China Evaluating the Safety and Efficacy of DEXTENZA® in Cataract Surgery Patients
SHANGHAI, May 31, 2023 /PRNewswire/ -- AffaMed Therapeutics ("AffaMed"), a global biotechnology company dedicated to developing and commercializing transformative pharmaceutical, digital and surgical products that address critical unmet medical needs in ophthalmological, neurological and psychiat...
Lynk Pharmaceuticals Completes 200 Million RMB Series C1 Financing to Accelerate Clinical Development of Core Products
HANGZHOU, China, May 31, 2023 /PRNewswire/ -- Lynk Pharmaceuticals Co., Ltd. (hereinafter referred to as 'Lynk Pharmaceuticals'), an innovative clinical stage company, today announced the successful completion of aRMB 200 million Series C1 financing round. There was joint participation by China G...
Excellent 12-Month Results from SELUTION SFA Trial Presented at JET
GENEVA, May 31, 2023 /PRNewswire/ -- 12-month results from the SELUTION SFA trial have been presented at the Japan Endovascular Treatment (JET) Conference inTokyo. The objective of this study was to assess the safety and efficacy of SELUTION SLR™, MedAlliance's novel sirolimus-eluting balloon, fo...
LZ901, A Recombinant Herpes Zoster Vaccine And The Core Product Of Luzhu Biotechnology, Completed Phase II Clinical Trail In Q2 2023
World's First Herpes Zoster Vaccine With A Tetrameric Molecular Structure BEIJING, May 30, 2023 /PRNewswire/ -- Beijing Luzhu Biotechnology Co., Ltd. ("Luzhu Biotechnology" or the "Company", together with its subsidiaries, the "Group", stock code: 02480.HK), a leading vaccine and antibody drug de...
Sun Pharma and Philogen enter into an Exclusive Distribution, License, and Supply Agreement for Commercializing specialty product, NIDLEGY™ in Europe, Australia and New Zealand
MUMBAI, India and SIENA, Italy, May 30, 2023 /PRNewswire/ -- Sun Pharmaceutical Industries Limited (Reuters: SUN.BO), (Bloomberg: SUNP IN), (NSE: SUNPHARMA), (BSE: 524715) (together with its subsidiaries and/or associated companies, "Sun Pharma") and Philogen S.p.A (BIT: PHIL) today announced tha...
Concept Medical's fourth IDE approval for the MagicTouch Sirolimus Coated Balloon is granted for the treatment of Superficial Femoral Artery Disease (SFA)
TAMPA, Fla., May 29, 2023 /PRNewswire/ -- The US FDA, on the 24th of May 2023,
granted an Investigational Device Exemption (IDE) approval forConcept Medical
Inc's
Hummingbird Bioscience Announces HMBD-002 Trials in Progress Poster at ASCO Annual Meeting 2023
* HMBD-002, a non-depleting, high-affinity anti-VISTA antibody, possesses key design features enabling robust anti-tumor activity in preclinical models, positioning it as a potentially important new therapy for VISTA-expressing cancers including triple-negative breast cancer and non-small cell ...
APPLICATION OF HOSPITAL-BASED HEALTH TECHNOLOGY ASSESSMENT: EXPERIENCE FROM THE WORLD AND SITUATION IN VIETNAM
HO CHI MINH CITY, Vietnam, May 29, 2023 /PRNewswire/ -- In the limited medical sources circumstance, the technology evaluation will help policymakers choose and establish the most appropriate health technology coordinating with conditions and actual occurrences, thereby ensuring the best medical ...
Menarini Group Shares New Analysis from EMERALD Clinical Study of ORSERDU® (Elacestrant) in Metastatic Breast Cancer at ASCO 2023
* ORSERDU (elacestrant) was approved by the FDA in January 2023 for estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer withESR1-mutations (ESR1-mut) that are found in up to 40% of tumors * In patients whose tumors har...
Antengene To Present Latest Results from TORCH-2 Study of ATG-008 in Advanced Solid Tumors in Poster Discussion at ASCO 2023
* The TORCH-2 study is a Phase I/II trial of the mTORC1/2 inhibitor ATG-008 plus the Anti-PD-1 monoclonal antibody toripalimab for the treatment of patients with advanced solid tumors * The combination treatment produced an objective response rate (ORR ) of 52.4% in the advanced cervical canc...
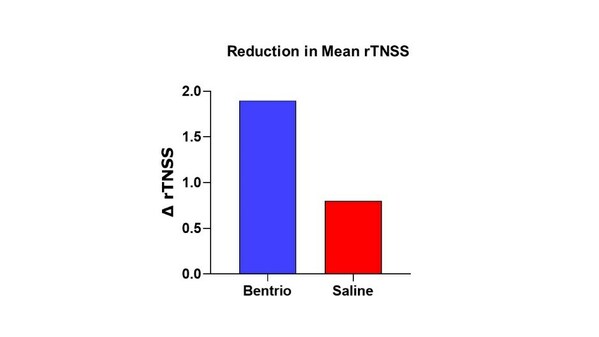
Altamira Therapeutics Reports Positive Top-Line Data from Bentrio Clinical Trial in Seasonal Allergic Rhinitis
* Bentrio® meets primary efficacy endpoint in NASAR clinical trial in seasonal allergic rhinitis * Clinically relevant and statistically significant improvement in Total Nasal Symptom Score over saline nasal spray control (p = 0.012) * Bentrio efficacy and tolerability rated as "good" or "ve...
Invivoscribe Announces Updated Reimbursement for the LeukoStrat CDx FLT3 Mutation Assay to Select Newly Diagnosed FLT3-ITD Positive AML Patients Eligible for VANFLYTA in Japan
SAN DIEGO, May 26, 2023 /PRNewswire/ -- Invivoscribe is pleased to announce that their LeukoStrat CDxFLT3 Mutation Assay® has received updated reimbursement byJapan's Ministry of Health, Labor and Welfare (MHLW) to aid in the selection of patients with newly diagnosedFLT3-ITD positive acute my...
AffaMed Therapeutics Announces Partner Allgenesis Reports Encouraging Preliminary Safety and Efficacy Data from the AG-73305 Phase 2a Trial for the Treatment of Diabetic Macular Edema
* AG-73305 was found to be safe and tolerable with no severe adverse effects (SAEs) after a single intravitreal injection of 0.5 mg and 1 mg in DME patients. * AG-73305 showed median improvements in Best Corrected Visual Accuity (BCVA) of 8.5 ETDRS letters with median Central Subfield Thickness...
Hope for knee osteoarthritis sufferers with advent of global clinical trial.
Paradigm Biopharma launches clinical trial website to support development
program for knee osteoarthritis therapy.
MELBOURNE, Australia, May 25, 2023 /PRNewswire/ -- With the launch of a new
website dedicated to their global clinical trials (https://hope4OA.com
Lynk Pharmaceuticals Announces First Cohort of Psoriatic Patients Dosed with LNK01004 in Phase Ib Clinical Study
HANGZHOU, China, May 25, 2023 /PRNewswire/ -- Lynk Pharmaceuticals Co., Ltd. (hereinafter referred to as 'Lynk Pharmaceuticals'), an innovative clinical stage company, announced that it has dosed the first cohort of psoriatic patients in a Phase Ib clinical trial of its innovative drug LNK01004. ...
Avixgen accelerates the progress on new drug pipeline development
- Pipeline development updates on age related macular degeneration, dry eye disease and atopic dermatitis SEOUL, South Korea, May 24, 2023 /PRNewswire/ -- DxVx has released development updates on the pipelines of its recently acquired company, Avixgen. DxVx acquired about 63% stake of Avixgen, w...
Allgenesis Announces Encouraging Preliminary Safety and Efficacy Data from the AG-73305 Phase 2a Trial for the Treatment of Diabetic Macular Edema
* AG-73305 was found to be safe and tolerable with no severe adverse effects (SAEs) after a single intravitreal injection of 0.5 mg and 1 mg in DME patients. * AG-73305 showed median improvements in Best Corrected Visual Accuity (BCVA) of 8.5 ETDRS letters with median Central Subfield Thickness...
Antengene Announces Claudin 18.2 Antibody-Drug Conjugate ATG-022 Granted Orphan Drug Designations by the U.S. FDA for the Treatment of Gastric and Pancreatic Cancers
SHANGHAI and HONG KONG, May 23, 2023 /PRNewswire/ -- Antengene Corporation Limited ("Antengene" SEHK: 6996.HK), a leading commercial-stage innovative, global biopharmaceutical company dedicated to discovering, developing and commercializing first-in-class and/or best-in-class medicines for hemato...
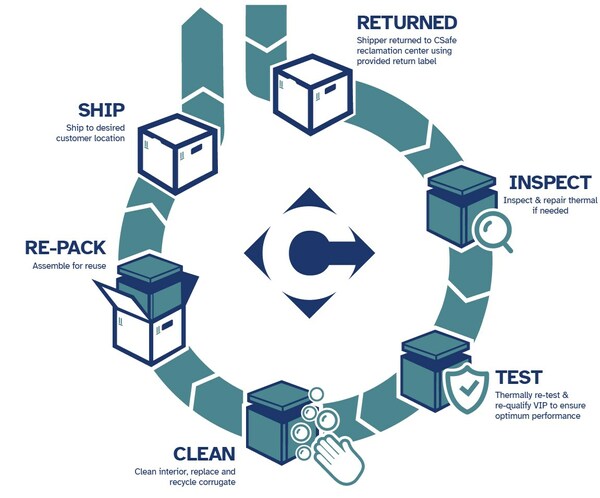
CSafe Providing Sustainable Thermal Protection for Vyjuvek Gene Therapy from Krystal Biotech
MONROE, Ohio, May 22, 2023 /PRNewswire/ -- CSafe, the largest provider of a complete range of active and passive temperature-controlled shipping solutions for the pharmaceutical industry, is honored to announce an innovative program forKrystal Biotech that provides the cold chain solution for Kry...
A random placebo-controlled clinical trial by CU Medicine shows that modulation of gut microbiome using oral microencapsulated live bacteria (SIM01) improves long COVID symptoms
HONG KONG, May 22, 2023 /PRNewswire/ -- 70% of patients in Hong Kong who have recovered from COVID-19continue to suffer from at least one long COVID symptoms at around 6 months. There is however no proven treatment for long COVID. The Chinese University of Hong Kong's (CUHK) Faculty of Medicine (C...
Week's Top Stories
Most Reposted
DBS is First Bank in Asia Pacific to Pilot Visa Intelligent Commerce for Everyday Payments
[Picked up by 319 media titles]
2026-02-16 10:00Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 316 media titles]
2026-02-19 14:30Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 277 media titles]
2026-02-17 19:12Kung Fu Meets Spring -- Unitree Spring Festival Gala Robots Present "Cyber Real Kung Fu" in the Year of the Horse
[Picked up by 256 media titles]
2026-02-17 14:16SMU MBA Rises in FT Global Rankings, Excelling in ESG, Salary and Value-for-Money
[Picked up by 251 media titles]
2026-02-16 08:00