Pharmaceuticals
Hummingbird Bioscience and Merck Enter Collaboration to Evaluate HMBD-001 In Squamous Non-Small Cell Lung Carcinoma
* Hummingbird Bioscience enters a clinical trial collaboration and supply agreement with Merck to evaluate HMBD-001, a potentially best-in-class anti-HER3 mAb, in combination with cetuximab, an anti-EGFR mAb, in sqNSCLC * HMBD-001 used as monotherapy and in combination with cetuximab has been ...
BOTTICELLI, THE INQUIETUDE AND THE BEAUTY - Menarini presents in Florence its latest art monograph, dedicated to the symbol of the Italian Renaissance
FLORENCE, Italy, May 15, 2023 /PRNewswire/ -- Successful artist, sensitive interpreter of the culture of his time and author of extraordinarily relevant works, Sandro Botticelli was a master of beauty. With his "Venuses," he unknowingly established a canon of perfection that closely reflects tha...
Rona Therapeutics and Keymed Biosciences Announce Collaboration to Discover and Develop First-in-class siRNA Therapeutics for Glomerulonephritis
SHANGHAI, May 15, 2023 /PRNewswire/ -- Rona Therapeutics, Inc ("Rona") and Keymed Biosciences ("Keymed", HKEX: 02162), today announced a collaboration to jointly discover and develop first-in-class siRNA therapeutics for Glomerulonephritis (also referred as severe kidney diseases). The collaborat...
Innovent Receives NMPA Breakthrough Designation for IBI351 (KRASG12C Inhibitor) as Monotherapy for Previously Treated Advanced Colorectal Carcinoma
ROCKVILLE, Md. and SUZHOU, China, May 15, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, metabolic, autoimmune, ophthalmology and...
Access to Novavax's COVID Vaccine Grows with Extended Interim Authorization for Adolescents in Singapore
GAITHERSBURG, Md., May 12, 2023 /PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX), a global company advancing protein-based vaccines with its novel Matrix-M™ adjuvant, today announced that the Singapore Health Sciences Authority (HSA) has granted extended interim authorization and the Ministry of Heal...
CRM Trial Connect 2023: Minister of Health Malaysia, Dr. Zaliha Mustafa visits Taimei Technology's booth
SHANGHAI, May 12, 2023 /PRNewswire/ -- Taimei Technology, the digital platform for life science industry, attendedCRM Trial Connect 2023 held at the InterContinental Kuala Lumpur fromMay 11 to May 12, 2023. During the event, Dr. Zaliha Mustafa, Minister of Health Malaysia visited Taimei Technolog...
Results of Phase I Clinical Study of Qilu Pharma's PD-1/CTLA-4 Bifunctional Antibody Iparomlimab/Tuvonralimab Published Online in Journal of Hematology & Oncology
JINAN, China, May 12, 2023 /PRNewswire/ -- On May 8, 2023, Journal of Hematology & Oncology (JHO, 2022 impact factor of 23.168) published the results of the Phase I clinical study of Qilu Pharma's immunotherapy bifunctional antibody, QL1706 (J Hematol Oncol. 2023May 8;16(1):50). This study is the...
Nefecon Included in the Reimbursement Drug List of "Beijing Puhui Health Insurance Program" as a First-in-Disease Therapy for IgA Nephropathy
SHANGHAI, May 12, 2023 /PRNewswire/ -- An imported version of Nefecon (budesonide) delayed release capsule has been added to the 2023 New Reimbursement Drug List of Specialized Medicines of the "Beijing Puhui Health Insurance Program." Nefecon is a first-in-disease treatment for adults with prim...
Results of Phase I Clinical Study of Qilu Pharma's PD-1/CTLA-4 Bifunctional Antibody Iparomlimab/Tuvonralimab Published Online in Journal of Hematology & Oncology
JINAN, China, May 11, 2023 /PRNewswire/ -- On May 8, 2023, the authoritative oncology journal, Journal of Hematology & Oncology (JHO, 2022 impact factor of 23.168) published the results of the Phase I clinical study of Qilu Pharma's immunotherapy bifunctional antibody, QL1706 (Title: First-in-hum...
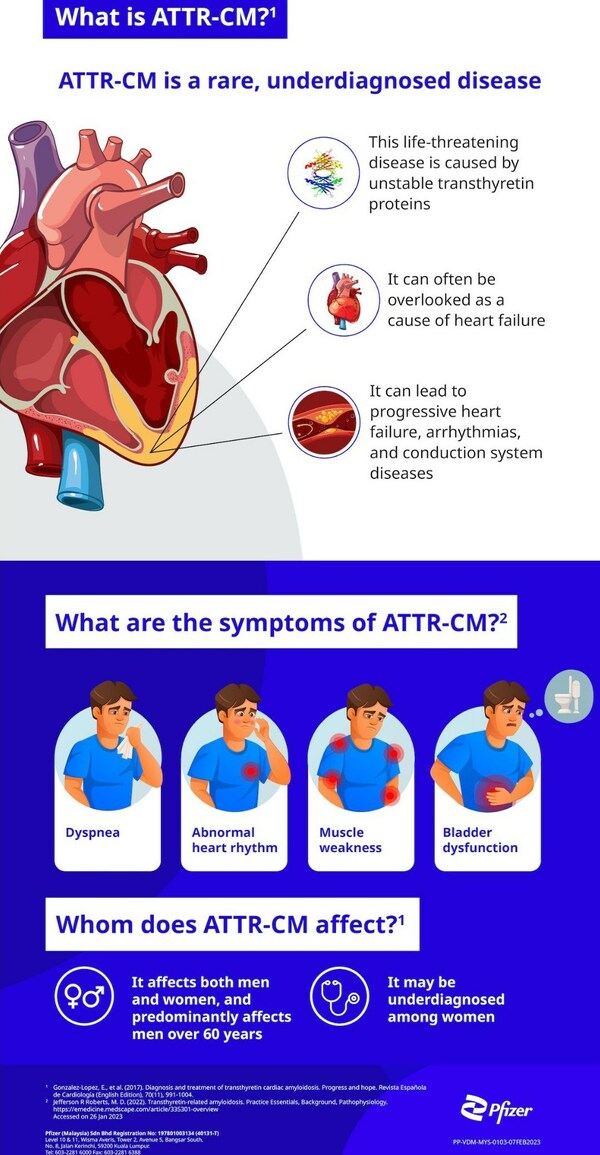
PFIZER CALLS FOR MORE AWARENESS OF TRANSTHYRETIN AMYLOID CARDIOMYOPATHY (ATTR-CM) - A RARE, LIFE-THREATENING DISEASE
KUALA LUMPUR, Malaysia, May 11, 2023 /PRNewswire/ -- In an effort to encourage people to take care of their heart health, Pfizer Malaysia is calling for Malaysians to learn more about transthyretin amyloid cardiomyopathy (ATTR-CM), a rare, life-threatening disease. ATTR-CM is caused by unstable ...
Nippon Express Italia Obtains IATA CEIV Pharma Certification for Its Facility near Milan Malpensa Airport
TOKYO, May 11, 2023 /PRNewswire/ -- Nippon Express Italia S.p.A. (hereinafter "NX Italia"), a group company of NIPPON EXPRESS HOLDINGS, INC., has acquired IATA CEIV Pharma certification*, a quality certificate for pharmaceutical transport established by the International Air Transport Association...
SHL Medical and MoonLake Immunotherapeutics collaborate to develop sonelokimab autoinjector
ZUG, Switerland, May 11, 2023 /PRNewswire/ -- SHL Medical, a world-leading provider of advanced drug delivery solutions, announced that it has signed a collaboration agreement with MoonLake Immunotherapeutics, a clinical-stage biotechnology company focused on creating next-level therapies for inf...
Innovent Announces Phase 2 Clinical Study of Higher dose 9 mg Mazdutide (IBI362) in Chinese Adults with Obesity Achieved the 24-Week Primary Endpoint
ROCKVILLIE, Md. and SUZHOU, China, May 11, 2023 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, metabolic, autoimmune, ophthalmology and ...
Servier receives European Commission approval of Tibsovo® (ivosidenib tablets) in IDH1-mutated Acute Myeloid Leukemia and IDH1-mutated Cholangiocarcinoma
* Marketing Authorization granted for Tibsovo® as the first and only approved IDH1 targeted therapyin Europe * IDH1-mutated Acute Myeloid Leukemia and IDH1-mutated Cholangiocarcinoma, difficult and hard-to-treat cancer PARIS, May 10, 2023 /PRNewswire/ -- Servier, a global pharmaceutical group...
Doceree forms an exclusive partnership with Hello Health Group to strengthen its global footprint; forays into 8 South East Asian markets
The partnership opens a first-of-its-kind service for Hello Health Group's
pharmaceutical clients,enabling them to target HCPs with precision and
efficiency
NEW DELHI, May 10, 2023 /PRNewswire/ -- Doceree
The China NMPA Approves TYVYT® (sintilimab injection) in Combination with Bevacizumab and Chemotherapy in Patients with EGFR-mutated Non-squamous NSCLC who Progressed after EGFR-TKI Therapy
ROCKVILLIE, Md. and SUZHOU, China, May 10, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of cancer, autoimmune, metabolic, ophthalmology and ...
Menarini Group Announces New Data on Elacestrant at 2023 ESMO Breast Cancer Congress and 2023 ASCO Annual Meeting
FLORENCE, Italy and NEW YORK, May 9, 2023 /PRNewswire/ -- The Menarini Group ("Menarini"), a leading Italian pharmaceutical and diagnostics company, and Stemline Therapeutics ("Stemline"), a wholly-owned subsidiary of the Menarini Group, announced today that they will present new data related to ...
Positive Phase 2 Topline Results Show Novavax's COVID-Influenza Combination, Stand-alone Influenza and High-dose COVID Vaccine Candidates Demonstrate Robust Immune Responses
* This Phase 2 trial is evaluating three vaccine candidates: COVID-Influenza Combination, stand-alone influenza and high-dose COVID * Preliminary topline immune responses for all three vaccine candidates were robust versus authorized comparators * For the stand-alone influenza vaccine candid...
Hummingbird Bioscience to Present Preclinical Proof of Concept for Potentially First-In-Class Antibody Targeting Autoimmune Diseases
* HMBD-011 is a potentially first-in-class anti-VH4-34 antibody developed for treatment of VH4-34+ antibody-driven pathogenesis in lupus and cold agglutinin disease * Data presented shows preclinical proof of concept for HMBD-011 clearance of VH4-34+ antibodies and effective inhibition of col...
Ascletis Announces China NMPA Approval of Conducting a Phase IIa Clinical Trial for ASC10 to Treat Respiratory Syncytial Virus Infection
--Respiratory syncytial virus (RSV) infection treatment remains huge unmet medical needs and there is no effective drug for treatment globally so far --Dosage of 800 mg ASC10, twice daily was selected to conduct a Phase IIa study in patients with RSV infection --Preclinical research showed that ...
Week's Top Stories
Most Reposted
Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 321 media titles]
2026-02-19 14:30NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 301 media titles]
2026-02-23 08:00Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 280 media titles]
2026-02-17 19:12Vitafoods Asia 2026 Expands by 30%: A Bigger, More Dynamic Trade Event with Exciting New Features & Increased International Participation
[Picked up by 277 media titles]
2026-02-23 10:09Kung Fu Meets Spring -- Unitree Spring Festival Gala Robots Present "Cyber Real Kung Fu" in the Year of the Horse
[Picked up by 259 media titles]
2026-02-17 14:16