Pharmaceuticals
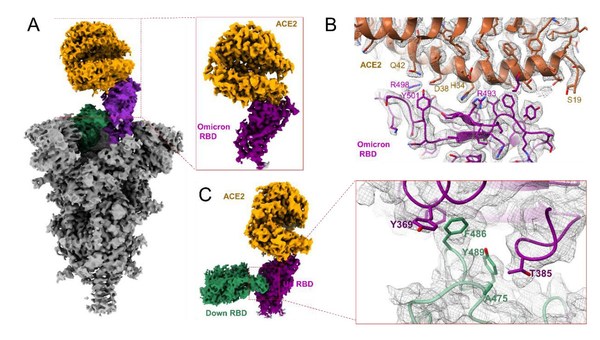
Breakthrough Discoveries on Novel Features of Omicron Variant and An Anti-Omicron Antibody JMB2002
SHANGHAI, Jan. 4, 2022 /PRNewswire/ -- Joint research results from Biologics of Jemincare and Shanghai Institute ofMateria Medica (SIMM) of Chinese Academy of Sciences (CAS) confirmed that JMB2002, an anti-SARS-CoV-2 neutralizing antibody (NAb) discovered by Biologics of Jemincare is still effect...
Everest Medicines Announces Taiwan FDA Has Accepted New Drug Application for Sacituzumab Govitecan in Second-Line Metastatic Triple-Negative Breast Cancer
SHANGHAI, Jan. 4, 2022 /PRNewswire/ -- Everest Medicines
Sihuan Pharmaceutical (0460.HK): Sunshine Life Insurance Leads Investment in Xuanzhu Biopharm
HONG KONG, Jan. 3, 2022 /PRNewswire/ -- Sihuan Pharmaceutical Holdings Group Ltd. (the "Company" or "Sihuan Pharmaceutical", together with its subsidiaries, collectively referred to as the "Group"; Hong Kong Stock Exchange stock code: 0460) is pleased to announce that, its subsidiary Xuanzhu...
Ascletis Expands Ritonavir Oral Tablet Production and Announces Oral Direct-Acting Antiviral Pipeline Against SARS-CoV-2 Virus
- Ritonavir oral tablet annual production capacity has been expanded to 100 million tablets and can be further rapidly expanded based on market demand - ASC10 is an oral direct-acting antiviral drug candidate targeting RNA dependent RNA polymerase (RdRp) to treat SARS-CoV-2 infection - ASC11 is ...
CStone Pharmaceuticals announced the IND approval of CS5001, a potential global best-in-class ROR1-targeting ADC by the U.S. Food and Drug Administration
SUZHOU, China, Jan. 3, 2022 /PRNewswire/ -- CStone Pharmaceuticals ("CStone", HKEX: 2616), a leading biopharmaceutical company focused on the research, development, and commercialization of innovative immuno-oncology therapies and precision medicines, today announced that the investigational new ...
Seegene Airlifts 1.7 million COVID-19 Tests to Israel Battling the Omicron Variant by a charter plane
* On December 29, Seegene to deliver Allplex™ SARS-CoV-2 Master Assay that can preemptively detect the Omicron variant at the primary screening stage * Earlier this month, the company has hired a private plane to deliver 2.8 million COVID-19 diagnostic tests to European countries includingItal...
DoctorOnCall Offers Free Medical Assistance To Flood Victims
KUALA LUMPUR, Malaysia, Dec. 29, 2021 /PRNewswire/ -- Amidst Malaysia's worst flash flooding following heavy rains over the weekend that displaced tens of thousands of people,Malaysia's first and largest digital health platform, DoctorOnCall, has announced a flood relief medical assistance initia...
VISTA Eye Specialist resolved to be a Changemaker and A Force for Good with B Corp Certification.
PETALING JAYA, Malaysia, Dec. 29, 2021 /PRNewswire/ -- VISTA Eye Specialist (VISTA) believes that being one of the leading eye specialists is not just about medical care. "As part of our mission statement, we believe that other than to safely help people see their best, our goal is about changing...
Muslim-Friendly Malaysia Showcases Healthcare Excellence at Expo 2020 Dubai
DUBAI, UAE, Dec. 28, 2021 /PRNewswire/ -- Malaysia Healthcare, will be showcasing its excellence in healthcare service delivery and providing seamless healthcare journey experiences during Week 14 of Expo 2020 Dubai. From 2nd - 8th January 2022, expo participants can visit the Malaysia Pavilion to...
CStone announced new drug approval of precision therapy AYVAKIT® (avapritinib) in Hong Kong, China for the treatment of PDGFRA D842V mutant gastrointestinal stromal tumors (GIST)
* AYVAKIT is the first precision therapy approved in Hong Kong, China for the treatment of patients with PDGFRA D842V mutant GIST * This is CStone's fifth NDA approval in Greater China this year SUZHOU, China ,Dec. 28, 2021 /PRNewswire/ -- CStone Pharmaceuticals ("CStone", HKEX: 2616), a leadi...
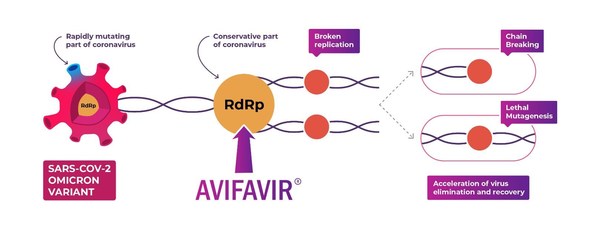
ChemRar Group announces the Russian Avifavir® drug is effective against variants of COVID-19, including Delta and Omicron
Avifavir® is effective against various variants of coronavirus, including Delta and Omicron, as it affects the highly conservative and mutation-resistant replication systems of RNA virus (RdRp). The virus is incapable of developing resistance to favipiravir even with long-term exposure on infect...
Brii Bio Doses First Patient in Phase 2a/2b Clinical Trial of BRII-179 (VBI-2601) for the Functional Cure of Chronic Hepatitis B
DURHAM, N.C. and BEIJING, Dec. 27, 2021 /PRNewswire/ -- Brii Biosciences
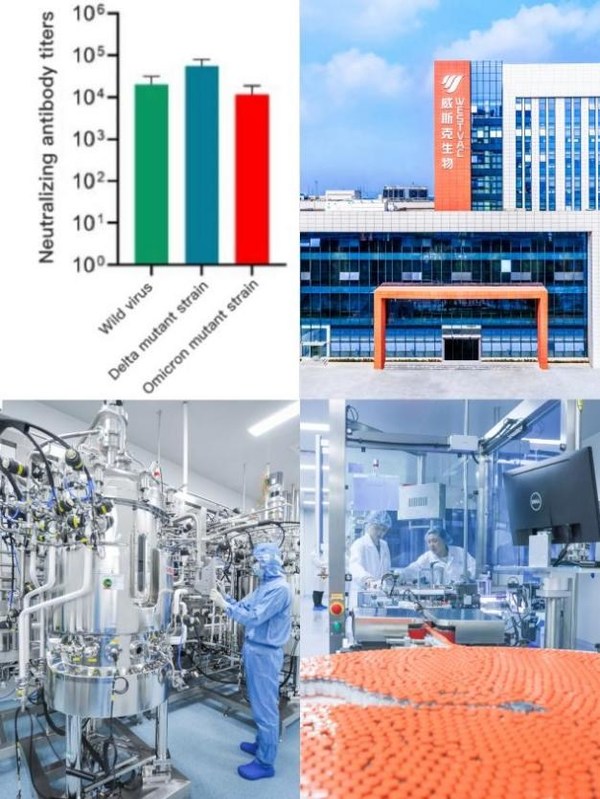
COVID-19 Vaccine Developed by WestVac BioPharma Has High Titer Neutralizing Antibodies Against Omicron
CHENGDU, China, Dec. 24, 2021 /PRNewswire/ -- WestVac BioPharma Co., Ltd. has achieved a significant progress in the second generation COVID-19 vaccine against the Omicron mutant strain. The vaccine belongs to the latest fifth generation vaccine technology, targeting the S-RBD protein of the COV...
Innovent Announces NMPA of China Acceptance of a Supplemental New Drug Application for TYVYT® (Sintilimab Injection plus Bevacizumab Biosimilar Injection and Chemotherapy in Patients with EGFR-mutated Non-squamous Non-small Cell Lung Cancer who Progressed after EGFR-TKI Therapy
SAN FRANCISCO and SUZHOU, China, Dec. 24, 2021 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of cancer, metabolic, autoimmune and other major diseas...
Novavax and SK bioscience Expand Manufacturing Agreement
- Novavax secures additional manufacturing capacity through 2022 - SK bioscience secures long-term license to supply NVX-CoV2373 for the Korean market GAITHERSBURG, Md., Dec. 24, 2021 /PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX), a biotechnology company dedicated to developing and commercializin...
Novo Nordisk BASEA office certified Best Place to Work for 2021
KUALA LUMPUR, Malaysia, Dec. 23, 2021 /PRNewswire/ -- Novo Nordisk BASEA office, part of the global healthcare company with more than 95 years of innovation and leadership in diabetes care, has recently achieved the Best Place to Work certification for 2021. During the assessment, the organizati...
Alphamab Oncology Announced First Patient Dosed in the U.S. in A Phase II Pivotal Clinical Study of KN046
SUZHOU, China, Dec. 23, 2021 /PRNewswire/ -- Alphamab Oncology (stock code: 9966 HK) announced dosing of the first patient of its proprietary PD-L1/CTLA-4 bispecific antibody KN046 inthe United States. The patient received treatment in ENREACH-Thymic, a phase II, open label, multi-center pivotal...
Lion TCR Receives FDA Fast Track Designation for its HBV-specific TCR T Cell Therapy for Hepatocellular Carcinoma
SINGAPORE and GUANGZHOU, China and LOS ANGELES, Dec. 23, 2021 /PRNewswire/ -- Lion TCR Pte Ltd today announced that it has received Fast Track Designation from United States Food and Drug Administration (U.S. FDA) for LioCyx-M004, autologous T-cells transfected with mRNA encoding Hepatitis B surf...
LINEPHARMA INTERNATIONAL FILES FOR MANUFACTURING, MARKETING APPROVAL OF THE ABORTION PILL IN JAPAN
LONDON and TOKYO, Dec. 23, 2021 /PRNewswire/ -- Linepharma International Ltd., a global leader in medical abortion, announced today that its subsidiary Linepharma KK has applied for manufacturing and marketing approval inJapan for its oral drug MEFEEGO™ for the medical termination of pregnancies ...
Karyopharm and Menarini Group Enter into Exclusive License Agreement to Commercialize NEXPOVIO® (selinexor) in Europe and Other Key Global Territories
- Menarini Group Obtains Exclusive Rights to Commercialize NEXPOVIO for the Treatment of Hematologic and Solid Tumor Oncology Indications inEurope (including theUnited Kingdom), Latin America and Other Key Countries - Karyopharm to Receive $75 Million Upfront, then Eligible to Receive Up to $202...
Week's Top Stories
Most Reposted
LINKDOOD Breaks Language Barriers, Ushering a New Era for Cross-Border Romance
[Picked up by 326 media titles]
2024-04-29 06:00Multiple achievements made in China-Hungary BRI conference
[Picked up by 310 media titles]
2024-05-03 06:59Dow showcases circular and innovative materials science solutions and industry collaborations at Chinaplas 2024
[Picked up by 293 media titles]
2024-04-30 10:11Xinhua president, Hungarian economy minister vow to bolster media cooperation
[Picked up by 281 media titles]
2024-05-03 06:25Puyuan Fashion Resort 2024: A Grand Unveiling of Global Trends and Local Heritage
[Picked up by 269 media titles]
2024-04-28 09:39