Pharmaceuticals
Telix Completes Acquisition of Next-Generation Therapeutic Assets and Innovative Biologics Technology Platform
MELBOURNE, Australia and INDIANAPOLIS, Jan. 31, 2025 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Nasdaq: TLX, Telix, the Company) today announces it has completed the acquisition from antibody engineering company ImaginAb, Inc. (ImaginAb). The acquisition includes a pipeline of next...
A Total of USD 12.7 Million Investment in New Diagnostics and Drug Development for NTDs and Malaria to Partners Including the Ohio State University, PATH, GSK and Others
TOKYO, Jan. 30, 2025 /PRNewswire/ -- The Global Health Innovative Technology (GHIT) Fund announced today a total investment of approximatelyJPY 2 billion ( USD 12.7 million1) in eight projects for the development of new diagnostics and drugs for neglected tropical diseases (NTDs) and malaria.2 J...
Asia Book of Records Honors Cipla (Emerging Markets & Europe business unit) for Record-Breaking Doctor engagement on COPD Awareness Initiative
Cipla's (EMEU BU) 'Take Charge – I Pledge' campaign engaged 4,350 doctors across six countries, setting a new benchmark in raising COPD awareness. MUMBAI, India, Jan. 29, 2025 /PRNewswire/ -- Cipla Limited (BSE: 500087) (NSE: CIPLA EQ) referred to as "Cipla" today announced that itsEmerging Marke...
Telix Completes Acquisition of RLS (USA) Inc.
MELBOURNE, Australia and INDIANAPOLIS, Jan. 28, 2025 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Nasdaq: TLX, Telix, the Company) today announces it has completed the acquisition of RLS (USA) Inc. (RLS; RLS Radiopharmacies), America's only Joint Commission-accredited radiopharmacy n...
CARiNG Pharmacy Celebrates Consecutive Gold Triumph at the 2024 Putra Aria Brand Awards
KUALA LUMPUR, Malaysia, Jan. 27, 2025 /PRNewswire/ -- CARiNG Pharmacy is thrilled to announce its back-to-back victory in securing the Gold award in the retail category at the 2024 Putra Aria Brand Awards. This recognition highlights the brand's unwavering commitment to excellence in the retail ...
X4 Pharmaceuticals Announces EMA Validation of Marketing Authorization Application (MAA) for Mavorixafor - licenced to Norgine for commercialisation in Europe.
LONDON, Jan. 25, 2025 /PRNewswire/ -- Following the licensing agreement with X4 Pharmaceuticals, Norgine is pleased to see the announcement from X4 today that their Marketing Authorization Application (MAA) for mavorixafor for the treatment of WHIM syndrome (warts, hypogammaglobulinemia, infectio...
Kelun-Biotech's Product Tagitanlimab Approved for Marketing in Second Indication in Combination with Cisplatin and Gemcitabine For the First-line Treatment of Patients with recurrent or metastatic NPC
CHENGDU, China, Jan. 23, 2025 /PRNewswire/ -- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (the "Company") announced that the Company received marketing authorization inChina from National Medical Products Administration (NMPA) for the programmed cell death ligand 1(PD-L1)-directed innovativ...
GC Cell Symposium with Bifarma Expands Global Impact and Reach for Immuncell-LC
YONGIN, South Korea, Jan. 23, 2025 /PRNewswire/ -- GC Cell
TraceLink Named to the 2025 RXinsider Pharmacy500 List for Excellence in Pharmacy Supply Chain Innovation
TraceLink recognized for advancing digitalization and orchestration in the pharmacy supply chain, ensuring safe and timely access to medications BOSTON, Jan. 22, 2025 /PRNewswire/ -- TraceLink, the largest end-to-end digital network platform for intelligent supply chain orchestration, is honored ...
JelloX Aims to Improve Patient-Centered Care with AI-powered 3D Digital Pathology
First shared at a recent Japanese Society of Digital Pathology meeting, the
company is forging a global alliance with a leading US medical institution and
other organizations to harness technology for better patient care and outcomes
in oncology.
TAIPEI, Jan. 22, 2025 /PRNewswire/ -- JelloX
Lepu Biopharma has granted ArriVent Exclusive License for MRG007, a Potential Best-in-Class ADC for the treatment of Gastrointestinal Cancers
HONG KONG, Jan. 22, 2025 /PRNewswire/ -- Today, Lepu Biopharma Co., Ltd (Stock Code: 02157.HK) announced that it has entered into an exclusive licensing agreement withArriVent BioPharma, Inc. for MRG007, a Potential Best-in-Class antibody- drug conjugate ("ADC") for the treatment of Gastrointesti...
Ascletis Announces Positive Results from U.S. Phase Ia Single Ascending Dose Study of Small Molecule Oral GLP-1R Agonist ASC30 and Provides Program Update
* ASC30 oral tablet demonstrated dose-proportional pharmacokinetic (PK) properties and a long half-life (t1/2) up to 60 hours in the single ascending dose (SAD) study of patients with obesity, supporting once-daily or less frequent oral dosing. * ASC30 oral tablet was generally safe and well ...
MedAdvisor Solutions' helps pharmacists in Australia deliver more than 115,000 clinical services to patients through Expanded Scope of Practice Pilots in 2024
New case study showcases improved operational efficiency and patient care with enhanced workflow and clinical services for pharmacists MELBOURNE, Australia, Jan. 21, 2025 /PRNewswire/ -- MedAdvisor Solutions, a global leader in patient engagement and pharmacy technology, published its newest cas...
SIRPAD - WORLD'S LARGEST RCT INVESTIGATING MAGICTOUCH PTA SIROLIMUS COATED BALLOON COMPLETES PATIENT ENROLMENT
TAMPA, Fla., Jan. 20, 2025 /PRNewswire/ -- Concept Medical Inc
Innovent and ASK Pharm Jointly Announce NMPA Approval of Limertinib, a Third-generation EGFR TKI for the Treatment of Lung Cancer
SAN FRANCISCO and SUZHOU, China, Jan. 17, 2025 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncologic, autoimmune, cardiovascular and metaboli...
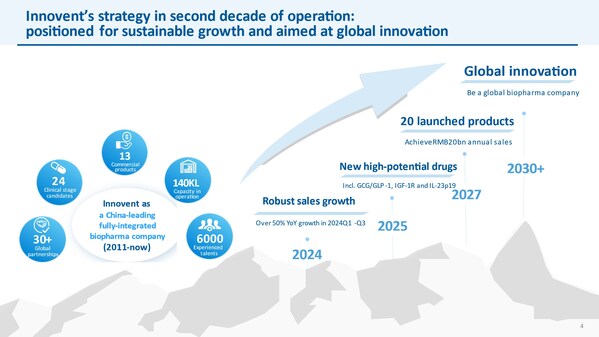
Innovent Presents at the 43rd Annual J.P. Morgan Healthcare Conference
Advancing into new stage of sustainable growth and global innovation SAN FRANCISCO and SUZHOU, China, Jan. 17, 2025 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines ...
Illuccix® Receives European Approval
MELBOURNE, Australia and LIÈGE, Belgium, Jan. 17, 2025 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Nasdaq: TLX, Telix, the Company) today announces that it has received a positive decision on the Marketing Authorization Application (MAA) for its prostate cancer PET[1] imaging agent ...
Concept Medical Announces First Patient Enrollment in the MAGICAL BTK IDE Trial, Launching its PAD Clinical Program in the United States
TAMPA, Fla., Jan. 17, 2025 /PRNewswire/ -- Concept Medical Inc.
SEACare 2025 - Your Gateway to Southeast Asia's Thriving Healthcare Market and Strategic Connections
Unlocking Opportunities in Southeast Asia's Healthcare Market KUALA LUMPUR, Malaysia, Jan. 16, 2025 /PRNewswire/ -- SEACare 2025, the Southeast Asia Healthcare & Pharma Show, returns for its 25th edition from 23 to 25 April 2025 at the Malaysian International Trade and Exhibition Centre (MIT...
BGI Genomics Empowers Brunei's National Cervical Cancer Prevention Program
SHENZHEN, China, Jan. 16, 2025 /PRNewswire/ -- On January 15, 2025, the Brunei Ministry of Health (MOH), in partnership with Borneo Genomics Innovation (BGIB), a joint venture of BGI Genomics inBrunei, launched the National Cervical Cancer Prevention and Control Program along with the 2025 Nation...
Week's Top Stories
Most Reposted
Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 328 media titles]
2026-02-19 14:30Never Miss a Message: Agoda's Customer Support Now Travels With You
[Picked up by 327 media titles]
2026-02-24 12:00NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 311 media titles]
2026-02-23 08:00Klook and Osaka Convention & Tourism Bureau sign MoU to advance inbound tourism and foster socio-economic development throughout Osaka Prefecture
[Picked up by 302 media titles]
2026-02-24 16:13Vitafoods Asia 2026 Expands by 30%: A Bigger, More Dynamic Trade Event with Exciting New Features & Increased International Participation
[Picked up by 288 media titles]
2026-02-23 10:09