Pharmaceuticals
Lepu Biopharma has granted ArriVent Exclusive License for MRG007, a Potential Best-in-Class ADC for the treatment of Gastrointestinal Cancers
HONG KONG, Jan. 22, 2025 /PRNewswire/ -- Today, Lepu Biopharma Co., Ltd (Stock Code: 02157.HK) announced that it has entered into an exclusive licensing agreement withArriVent BioPharma, Inc. for MRG007, a Potential Best-in-Class antibody- drug conjugate ("ADC") for the treatment of Gastrointesti...
Ascletis Announces Positive Results from U.S. Phase Ia Single Ascending Dose Study of Small Molecule Oral GLP-1R Agonist ASC30 and Provides Program Update
* ASC30 oral tablet demonstrated dose-proportional pharmacokinetic (PK) properties and a long half-life (t1/2) up to 60 hours in the single ascending dose (SAD) study of patients with obesity, supporting once-daily or less frequent oral dosing. * ASC30 oral tablet was generally safe and well ...
MedAdvisor Solutions' helps pharmacists in Australia deliver more than 115,000 clinical services to patients through Expanded Scope of Practice Pilots in 2024
New case study showcases improved operational efficiency and patient care with enhanced workflow and clinical services for pharmacists MELBOURNE, Australia, Jan. 21, 2025 /PRNewswire/ -- MedAdvisor Solutions, a global leader in patient engagement and pharmacy technology, published its newest cas...
SIRPAD - WORLD'S LARGEST RCT INVESTIGATING MAGICTOUCH PTA SIROLIMUS COATED BALLOON COMPLETES PATIENT ENROLMENT
TAMPA, Fla., Jan. 20, 2025 /PRNewswire/ -- Concept Medical Inc
Innovent and ASK Pharm Jointly Announce NMPA Approval of Limertinib, a Third-generation EGFR TKI for the Treatment of Lung Cancer
SAN FRANCISCO and SUZHOU, China, Jan. 17, 2025 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncologic, autoimmune, cardiovascular and metaboli...
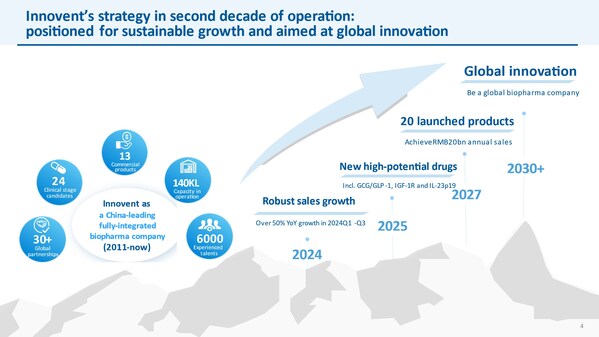
Innovent Presents at the 43rd Annual J.P. Morgan Healthcare Conference
Advancing into new stage of sustainable growth and global innovation SAN FRANCISCO and SUZHOU, China, Jan. 17, 2025 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines ...
Illuccix® Receives European Approval
MELBOURNE, Australia and LIÈGE, Belgium, Jan. 17, 2025 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Nasdaq: TLX, Telix, the Company) today announces that it has received a positive decision on the Marketing Authorization Application (MAA) for its prostate cancer PET[1] imaging agent ...
Concept Medical Announces First Patient Enrollment in the MAGICAL BTK IDE Trial, Launching its PAD Clinical Program in the United States
TAMPA, Fla., Jan. 17, 2025 /PRNewswire/ -- Concept Medical Inc.
SEACare 2025 - Your Gateway to Southeast Asia's Thriving Healthcare Market and Strategic Connections
Unlocking Opportunities in Southeast Asia's Healthcare Market KUALA LUMPUR, Malaysia, Jan. 16, 2025 /PRNewswire/ -- SEACare 2025, the Southeast Asia Healthcare & Pharma Show, returns for its 25th edition from 23 to 25 April 2025 at the Malaysian International Trade and Exhibition Centre (MIT...
BGI Genomics Empowers Brunei's National Cervical Cancer Prevention Program
SHENZHEN, China, Jan. 16, 2025 /PRNewswire/ -- On January 15, 2025, the Brunei Ministry of Health (MOH), in partnership with Borneo Genomics Innovation (BGIB), a joint venture of BGI Genomics inBrunei, launched the National Cervical Cancer Prevention and Control Program along with the 2025 Nation...
Innovent Receives NMPA Breakthrough Therapy Designation for IBI343 (Anti-CLDN18.2 ADC) as Monotherapy for Advanced Pancreatic Cancer
SAN FRANCISCO and SUZHOU, China, Jan. 16, 2025 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, cardiovascular and metabolic, autoimmune...
Samsung Biologics presents business updates at 2025 J.P. Morgan Healthcare Conference
- The company to mark new phase of growth at Bio Campus II with the opening of Plant 5 in April - Samsung Biologics to offer ADC services and invest in advanced development and manufacturing capabilities to better address clients' needs INCHEON, South Korea, Jan. 15, 2025 /PRNewswire/ -- Samsung...
Axcynsis Therapeutics Receives FDA Clearance for IND Application of AT03-65, a Differentiated CLDN6-Targeting ADC, Powered by AxcynDOT™ Technology
AT03-65 is the first program utilizing AxcynDOT™, a proprietary payload with a differentiated mechanism of action, to enter clinical development SINGAPORE, Jan. 15, 2025 /PRNewswire/ -- Axcynsis Therapeutics Pte Ltd ("Axcynsis"), a privately held biopharmaceutical company specialized in deliveri...
Telix Presentation to the 43rd Annual J.P. Morgan Healthcare Conference
MELBOURNE, Australia and INDIANAPOLIS, Jan. 14, 2025 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Nasdaq: TLX, Telix, the Company) today advises that Dr.Christian Behrenbruch, Managing Director and Group CEO, will be presenting this week at the 43rd Annual J.P. Morgan Healthcare Confe...
BioCina and NovaCina Merger Bolsters Global CDMO Industry
ADELAIDE, South Australia and PERTH, Western Australia, Jan. 14, 2025 /PRNewswire/ -- Global Contract Development and Manufacturing Organizations (CDMOs) BioCina and NovaCina announced a strategic merger that will create a powerful brand in biopharmaceutical and small molecule contract manufactur...
Dx&Vx Presents a New Paradigm for Next-Generation Infectious Disease Response with the Development of a Universal Vaccine
- Preparing for Phase 2 Global Trials of a Ferritin Platform-Based Virus-Like Particle Universal Coronavirus Vaccine - Based on the Excellent Safety and Immune Response in Phase 1 Clinical Results, with a Plan to Extend Administration Routes and Indications SEOUL, South Korea, Jan. 13, 2025 /PRNe...
CBC Group's R-Bridge Announces US$40M Synthetic Royalty-Backed Financing for Mirxes to Advance Global Expansion
- R-Bridge will provide bespoke financing of US$40 million with a synthetic royalty structure and flexible repayment terms - Financing will accelerate Mirxes, Singapore -based miRNA tech company's innovation and commercialization of cancer early detection solutions in new markets - Transacti...
Telix Exceeds FY24 Guidance with US$142M Q4 Revenue
MELBOURNE, Australia and INDIANAPOLIS, Jan. 13, 2025 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Nasdaq: TLX, Telix, the Company) today provides an update on its commercial and operational performance for the quarter ended31 December 2024 (Q4 2024). Sustained revenue growth * Q...
Scintimun® Commercialization Partnership with Curium Pharma
MELBOURNE, Australia and LIÈGE, Belgium, Jan. 13, 2025 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX; Nasdaq: TLX, Telix, the Company) today announces that it has entered into an agreement with Curium Pharma for the transfer of marketing and distribution rights for Scintimun® (99m Tc-be...
Telix to Acquire Next-Generation Therapeutic Assets and Innovative Biologics Technology Platform
MELBOURNE, Australia and INDIANAPOLIS, Jan. 13, 2025 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Nasdaq: TLX, Telix, the Company) today announces it has entered into an asset purchase agreement with antibody engineering company ImaginAb, Inc. (ImaginAb) to acquire a pipeline of next...
Week's Top Stories
Most Reposted
Never Miss a Message: Agoda's Customer Support Now Travels With You
[Picked up by 328 media titles]
2026-02-24 12:00HBX Group and Traveloka expand strategic partnership to deepen APAC supply and accelerate global distribution
[Picked up by 310 media titles]
2026-02-26 09:30Amadeus acquires SkyLink to accelerate the deployment of AI in travel
[Picked up by 308 media titles]
2026-02-26 19:57Klook and Osaka Convention & Tourism Bureau sign MoU to advance inbound tourism and foster socio-economic development throughout Osaka Prefecture
[Picked up by 302 media titles]
2026-02-24 16:13SMU and Fudan Launch Region's First Tech-Focused DBA
[Picked up by 300 media titles]
2026-03-02 09:15