Medical/Pharmaceuticals
Nuctech anti-epidemic sampling isolation cabin and mobile cabin help nucleic acid testing in hospitals of Beijing
BEIJING, July 3, 2020 /PRNewswire/ -- Recently, the temperature in Beijing has
been high. Every day, nucleic acid testing medics work outdoors wearing
protective clothing and sweating, which is admirable and distressing.
New strategic partnership to develop next-generation T cell detection reagents
SHANGHAI, China and COPENHAGEN, Denmark, July 2, 2020 /PRNewswire/ -- Today two MedTech companies, Polaris Biology ofChina and Tetramer Shop of Denmark, announce a strategic partnership to co-develop and distribute technologies that accelerate T cell science. First on the agenda is to commerciali...
WellsCare to target Middle Eastern market for its homecare laser pain treatment device
SEOUL, South Korea, July 2, 2020 /PRNewswire/ -- WellsCare, a member company of the Born2Global Centre, will be expanding its presence in theMiddle East starting in July. WellsCare has been an active member company of theBorn2Global Centre since 2019.   WellsCare recently signe...
Antengene Corporation Appoints Former BMS Senior Leader of Biostatistics Zhinuan YU as Corporate Vice President of Biometrics and Regulatory Enabling Functions
SHANGHAI and PHILADELPHIA, July 1, 2020 /PRNewswire/ --Antengene Corporation today announced its appointment of Zhinuan Yu, Ph.D., as Corporate Vice President (CVP) of Biometrics and Regulatory Enabling Functions. Zhinuan will be responsible for providing statistical leadership and strategic r...
FibroGenesis Identifies Mechanism Responsible for Blocking COVID19-Like Lung Inflammation
Regenerative Medicine Company Advances Development of Fibroblast-Based Product as an "Off the Shelf"Cell-Treatment for Coronavirus Acute Respiratory Distress Syndrome (ARDS) HOUSTON, July 1, 2020 /PRNewswire/ -- FibroGenesis announced today identification of molecular mechanisms associated with ...
Transcenta Announces First Patient Dosed in Phase I Clinical Trial of Claudin18.2 Targeting Monoclonal Antibody TST001 in the US
SUZHOU, China, July 1, 2020 /PRNewswire/ -- Transcenta Holding Limited ("Transcenta"), a global biotherapeutics company with fully-integrated capabilities in discovery, development and manufacturing of antibody-based therapeutics, announced today that the first subject has been dosed successfull...
Coronavirus Breakthrough: New Study Highlights Senhwa Biosciences Silmitasertib as Potential Treatment for COVID-19
TAIPEI and SAN DIEGO, July 1, 2020 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a clinical-stage biopharmaceutical company focused on Next Generation DNA Damage Response (DDR) therapeutics for the treatment of cancer, today announced that its drug Silmitasertib was again identified as o...
Anti-viral and Anti-Inflammatory Response: GoldenBiotech's Antroquinonol Receives FDA Approval on COVID-19 Phase 2 Trial in USA
TAIPEI, July 1, 2020 /PRNewswire/ -- Golden Biotechnology Corp. (GoldenBiotech, 4132.TWO), a leading Taiwanese biopharmaceutical company, announced that the FDA approved its investigational new drug (IND) application for a Phase II clinical trial of Antroquinonol (Hocena®) on COVID-19 patients in...
Immvira Announces the Appointment of Carl Yeung as Chief Financial Officer
SHENZHEN, China, June 30, 2020 /PRNewswire/ -- Today Immvira Group Company (Immvira or the "Company"), a biotechnology company focused on the development of new generation oncolytic viruses as potential cancer therapeutics, announces the appointment ofCarl Yeung as Chief Financial Officer, report...
'Curing Cancer without Surgery'
* "Successful disappearance of tumors in subjects upon administering the pain-free anticancer drug, Polytaxel, without side effects such as weight loss." * Suppressed 99.8% of tumors despite a non-toxic dose…more effective than existing FDA-approved pancreatic cancer drugs. * Hopes for a Kore...
Alterity Therapeutics meeting with US FDA provides development pathway for ATH434
MELBOURNE, Australia and SAN FRANCISCO, June 29, 2020 /PRNewswire/ -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) ("Alterity" or "the Company") is pleased to announce that it has received guidance from the US Food and Drug Administration (FDA) in relation to the development pathway for ATH434 ...
China Biologic Provides Additional Comments on the Xinjiang Deyuan and Shuanglin Transaction
BEIJING, June 29, 2020 /PRNewswire/ -- China Biologic Products Holdings, Inc. (NASDAQ: CBPO, "China Biologic" or the "Company"), a leading fully integrated plasma-based biopharmaceutical company inChina, today declared that the Company disagrees with the notice delivered by Xinjiang Deyuan Bioeng...
PharmAbcine Signs Long-term Contract Manufacturing Organization Agreement for Olinvacimab with Binex
DAEJEON, South Korea, June 26, 2020 /PRNewswire/ -- PharmAbcine (KRX: 208340) (CEO: Jin-san Yoo), biotech company focusing on the development of antibody therapeutics, announced on 18th that it had signed a contract manufacturing organization(CMO) agreement with biologics contract development ...
AUD$1.5M NHMRC Awards Grant to Expand Feasibility Trials for Stentrode, the First Interventional Neuromodulation Platform
Synchron's Stentrode brain-computer interface system is a fully-implantable therapeutic being evaluated for its safety and efficacy to restore functional independence via hands-free control of electronic devices in patients with upper limb paralysis SAN FRANCISCO and NEW YORK and MELBOURNE, June...
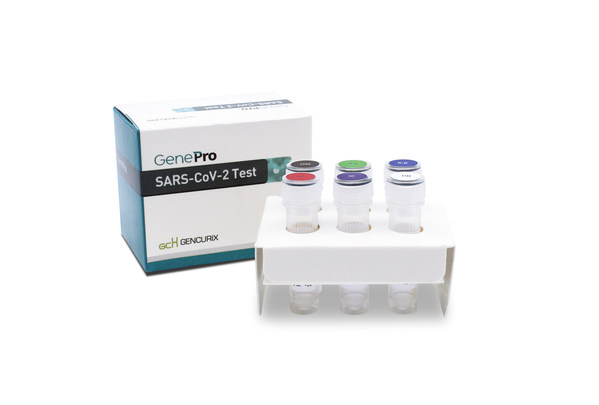
Gencurix wins FDA EUA for COVID-19 Test Kit
SEOUL, South Korea, June 24, 2020 /PRNewswire/ -- A Korean molecular diagnostic
company, Gencurix, Inc., has announced that they have received U.S. Food and
Drug Administration (FDA)'s Emergency Use Authorization (EUA) for its GenePro
SARS-CoV-2 Test.
VUNO Obtains CE Mark for 5 of Their Medical AI Solutions
SEOUL, South Korea, June 24, 2020 /PRNewswire/ -- Medical artificial
intelligence (AI) solution development companyVUNO Inc. and a member company of
theBorn2Global Centre, gained the Class IIa CE markings for five of their AI
solutions.
United Imaging Reaffirms Commitment to Cybersecurity by Partnering with Clearwater
HOUSTON, June 24, 2020 /PRNewswire/ -- United Imaging, a global leader in advanced medical imaging and radiotherapy equipment, has again affirmed its strategic commitment to leadership in cybersecurity risk management and cybersecurity protection by partnering with Clearwater, the leading provide...
Global Cord Blood Corporation to Report Fourth Quarter and Full Year Fiscal 2020 Financial Results
HONG KONG, June 24, 2020 /PRNewswire/ -- Global Cord Blood Corporation (NYSE: CO) (the "Company"),China's leading provider of cord blood collection, laboratory testing, hematopoietic stem cell processing and stem cell storage services, today announced that it plans to release financial results fo...
Mayne Pharma Announces FDA Filing Acceptance of New Drug Application for E4/DRSP in the US
ADELAIDE, Australia, June 24, 2020 /PRNewswire/ -- Mayne Pharma Group Limited (ASX: MYX) is pleased to announce the New Drug Application (NDA) for E4/DRSP to prevent pregnancy has been accepted for review by the US Food and Drug Administration (FDA). The FDA is expected to complete its review in ...
WAT Medical Delivers 650,000 Digital Thermometers to Canadian Customers
VANCOUVER, BC, June 22, 2020 /PRNewswire/ -- WAT Medical received an order for 650,000 digital thermometers from partners inCanada earlier this year. As of June, WAT Medical has finished manufacturing and delivering all of the requested units in a timely manner, while satisfying all of the specif...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 313 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00