Medical/Pharmaceuticals
Dr. Steven Yu Joins ChemPartner as Vice President of Regulated Bioanalysis
SHANGHAI, Sept. 17, 2020 /PRNewswire/ -- Shanghai ChemPartner announced today the appointment ofSteven Yu, Ph.D. as Vice President of Regulated Bioanalysis at the company headquarters inShanghai, China. Dr. Yu has more than 15 years of experience in drug development and extensive expertise in...
Expanding Their Existing Strategic Collaboration, GenScript ProBio Licensed Global Rights to Develop and Commercialize a SMAB Bispecific Antibody Molecule to REMD Biotherapeutics Inc.
NANJING, China, Sept. 17, 2020 /PRNewswire/ -- On September 16th, 2020, GenScript ProBio and REMD Biotherapeutics Inc. (REMD) announced that REMD has licensed a bispecific antibody derived from the Single-domain antibody fused to Monoclonal Ab (SMAB) platform developed by GenScript ProBio. REMD i...
Samsung Biologics signs development agreement with Panolos for solid tumor treatment
INCHEON, South Korea, Sept. 16, 2020 /PRNewswire/ -- Samsung Biologics (207940.KS) has entered into a service agreement with Panolos Bioscience to develop PB101, an Fc-fusion protein intended to treat solid tumors. Under this agreement, Samsung Biologics will provide a full scope of its developm...
Transcenta Successfully Scaled up the Continuous Perfusion Process and Completed GMP Manufacturing of a Novel Bispecific Antibody for Cancer Immunotherapy
SUZHOU, China and HANGZHOU, China, Sept. 15, 2020 /PRNewswire/ -- Transcenta, a global biotherapeutics company, today announced success in scaling up the continuous perfusion process to 200L and completion of GMP production of a bispecific antibody for a Phase 1 clinical study. "This is an impo...
Verkada Introduces Environmental Sensor to Provide Enhanced Visibility into Physical Spaces
Sensors seamlessly integrate with Verkada's enterprise video security and access control solutions to deliver real-time insights into buildings Verkada accelerates business growth in Q2 2020 with 65 percent quarter-over-quarter revenue growth amidst COVID-19 pandemic SAN MATEO, California, Sept....
PharmAbcine Unveils Olinvacimab's Positive Results from Phase Ib Combination Studies at KSMO 2020
DAEJEON, South Korea, Sept. 14, 2020 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks) today announced positive data from its two combination trials of olinvacimab, its leading clinical candidate in oncology, with MSD's pembrolizumab at the 13th Annual Meeting of the Korean Society of Medical O...
Coronavirus Breakthrough: Senhwa Reports First eIND Silmitasertib Treated Severe COVID-19 Patient - Discharged Following Five Days of Treatment
TAIPEI and SAN DIEGO, Sept. 11, 2020 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a clinical-stage biopharmaceutical company focused on next generation DNA Damage Response (DDR) therapeutics for the treatment of cancer, announced today that the first patient with severe COVID-19 demonst...
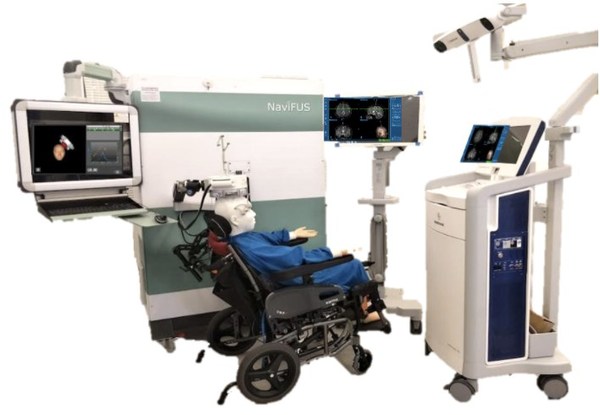
NaviFUS Launches Neuronavigation-guided Clinical Trial to Open the Blood-Brain-Barrier for Combination FUS-Bevacizumab Therapy in rGBM Patients
TAIPEI, Sept. 11, 2020 /PRNewswire/ -- Genovate Biotech (TPEX:4130) subsidiary andTaiwan-based focused ultrasound (FUS) manufacturer NaviFUS Corporation is pleased to announce the start of its clinical trial (NCT04446416) for the combination of FUS plus bevacizumab therapy. Researchers at Linkou ...
Tottenham Acquisition I Limited Announces Filing of a Registration Statement on Form S-4 in Connection with its Proposed Business Combination with Clene Nanomedicine, Inc.
NEW YORK, Sept. 10, 2020 /PRNewswire/ -- Tottenham Acquisition I Limited (Nasdaq: TOTA, TOTAU, TOTAW, TOTAR) ("Tottenham"), a publicly traded special purpose acquisition company, announced today that its subsidiary, Chelsea Worldwide Inc., has filed with the U.S. Securities and Exchange Commissio...
CMAB Biopharma and Laekna Therapeutics Enter Strategic Agreement for LAE005 Global Development and Commercialization Partnership
SUZHOU, China, Sept. 10, 2020 /PRNewswire/ -- CMAB Biopharma (Suzhou) Inc. ("CMAB"), and Laekna Therapeutics Shanghai Co., Ltd. ("Laekna Therapeutics"), today announced a strategic collaboration agreement in Suzhou BioBAY for speedup of Immune Checkpoint Inhibitor (ICI) drug candidate to the clin...
The Global Clinical CRO Fountain Medical Development and its Affiliates Rebrand as ClinChoice Inc
BEIJING and SHANGHAI, Sept. 9, 2020 /PRNewswire/ -- Fountain Medical Development (FMD K&L), together with its affiliates, is rebranding as ClinChoice Inc ("ClinChoice" or "Company"). ClinChoice is a global clinical stage Contract Research Organization ("CRO"), with over 1800 clinical research ...
Clarity Pharmaceuticals Announces the US FDA Grants Rare Paediatric Disease Designation to 64Cu-SARTATE™, a diagnostic for the clinical management of neuroblastoma
SYDNEY, Sept. 9, 2020 /PRNewswire/ -- Clarity Pharmaceuticals, a clinical-stage radiopharmaceutical company focused on the treatment of serious disease, is pleased to announce that the U.S. Food and Drug Administration (FDA) has granted Rare Paediatric Disease Designation (RPDD) to64Cu-SARTATE™, ...
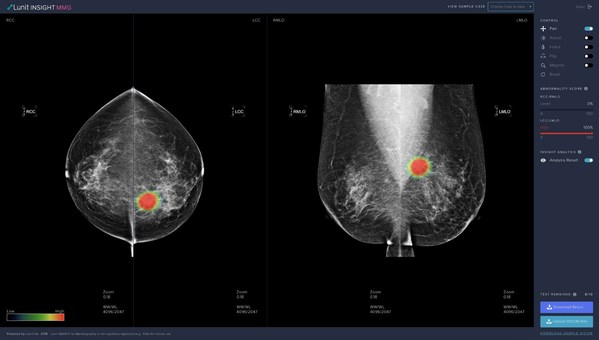
Recent Studies Reveal High Performance of Lunit AI in Breast Cancer Detection
SEOUL, South Korea, Sept. 9, 2020 /PRNewswire/ -- Recent studies have shown that Lunit's AI solution is as accurate as average radiologists when it comes to identifying breast cancer and it could potentially have a role as independent reader in the future, to reduce radiologist workload and possi...
NASDAQ Granted China SXT Pharmaceuticals, Inc. 180-Day Extension to Regain Compliance with Bid Price Requirement
TAIZHOU, China, Sept. 9, 2020 /PRNewswire/ -- China SXT Pharmaceuticals, Inc. (NASDAQ: SXTC) ("China SXT" or the "Company"), a specialty pharmaceutical company focusing on the research, development, manufacturing, marketing, and sales of Traditional Chinese Medicine Pieces ("TCMPs"), including Ad...
United Imaging Announces Research Collaboration with Massachusetts General Hospital Using Artificial Intelligence to Fight COVID-19
HOUSTON, Sept. 8, 2020 /PRNewswire/ -- United Imaging, a global leader in advanced medical imaging and radiotherapy equipment, announced today that its artificial intelligence (AI) teams inBoston and Shanghai will collaborate with Massachusetts General Hospital (MGH) to continue fighting COVID-19...
I-Mab Announces Upcoming Participation at September Conferences
SHANGHAI and GAITHERSBURG, Md., Sept. 8, 2020 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced its participation in the following conferences in Septem...
Transcenta Holding Announces Appointment of Dr. Charlie Qi as Senior Vice President of Global Clinical Development
SUZHOU, China, Sept.7, 2020 /PRNewswire/ -- Transcenta Holding Limited (Transcenta), a global biotherapeutics company with fully-integrated capabilities in discovery, development and manufacturing of antibody-based therapeutics, today announced the appointment of Dr.Charlie Qi as its Senior Vice...
Executive from Yuyu Pharma Receives Presidential Commendation for Gender Equality Employment
SEOUL, South Korea, Sept. 4, 2020 /PRNewswire/ -- Eugene Baik, Head of Production at Yuyu Pharma (CEORobert Wonsang Yu, KRX 000220), received the Presidential Commendation for contributions in leading the realization of gender equality employment. The award was given at the 2020 Gender Equality ...
I-Mab Announces $418 Million Private Placement with Hillhouse Capital-Led Consortium
SHANGHAI and GAITHERSBURG, Md., Sept. 4, 2020 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced that it has entered into definitive subscription agreeme...
AbbVie and I-Mab Enter into Global Strategic Partnership for Differentiated Immuno-oncology Therapy
NORTH CHICAGO, Illinois and SHANGHAI, China, Sept. 4, 2020 /PRNewswire/ -- AbbVie (NYSE: ABBV) and I-Mab (Nasdaq: IMAB) announced today that AbbVie and I-Mab have signed a broad, global collaboration agreement for the development and commercialization of lemzoparlimab (also known as TJC4), an inn...
Week's Top Stories
Most Reposted
LONG AN INTERNATIONAL PORT JOINS 12TH PORTECH ASIA SUMMIT 2025 IN MALAYSIA
[Picked up by 334 media titles]
2025-01-18 03:30Hong Kong Airlines Takes Off to Australia's Gold Coast Bringing Popular Travel Option for the Chinese New Year
[Picked up by 307 media titles]
2025-01-18 17:00HARD ROCK HOTEL BALI ACHIEVES PRESTIGIOUS GSTC CERTIFICATION
[Picked up by 304 media titles]
2025-01-20 14:27dss⁺ Announces Strategic Changes to Executive Leadership Team in Asia Pacific Accelerating growth and impact for high-hazard industries in the region
[Picked up by 302 media titles]
2025-01-21 16:00Infobip Research Unveils Opportunities for Brands to Engage Customers with Rich Communication Services (RCS) across APAC
[Picked up by 288 media titles]
2025-01-20 21:09