Medical/Pharmaceuticals
I-Mab Receives China CDE Approval to Initiate Phase 3 Clinical Trial of Eftansomatropin in Pediatric Patients with Growth Hormone Deficiency
SHANGHAI and GAITHERSBURG, Md., Sept. 30, 2020 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced that the China Center for Drug Evaluation (CDE) has app...
The 11th China (Taizhou) International Medical Expo ended successfully
NANJING, China, Sept. 30, 2020 /PRNewswire/ -- From September 19 to 21, the 11th China (Taizhou) International Medical Expo was held at the China Medical City Exhibition Center. With the theme of "Development Trends in the Medical and Health Industry under the Epidemic", the current medical expo h...
111, Inc. Builds Digitally-enabled, Omni-channel Drug Commercialization Platform as Part of its Goal to Provide Full Life-cycle Healthcare Management Solutions for Patients
SHANGHAI, Sept. 30, 2020 /PRNewswire/ -- On September 24, 2020, 111, Inc. ("111" or the "Company") (NASDAQ: YI), a leading digital healthcare platform dedicating to digitally connecting patients with drugs and healthcare services inChina, hosted its 3rd annual China Online Healthcare Summit and P...
TaiGen Biotechnology Out-Licensed Taigexyn (Nemonoxacin) to Luminarie Canada for Canada, Australia and New Zealand
TAIPEI, Sept. 30, 2020 /PRNewswire/ -- TaiGen Biotechnology Company, Limited ("TaiGen")(Taiwan: 4157) today announced that they have signed an exclusive licensing agreement with Luminarie Canada Inc. ("Luminarie"), a leading Canadian pharmaceutical company, to develop and commercialize Taigexyn® ...
JIECANG announced the official opening of the new headquarters at the 20th anniversary celebration
SHAOXING, China, Sept. 29, 2020 /PRNewswire/ -- On September 28, JIECANG celebrated its 20th anniversary in JIECANG Life and Health Industrial Park in Zhejiang. With its customer-centric strategy, strong product customization capabilities and production capacity, two decades of focus in linear mot...
Cardiac Dimensions Announces $17.5 Million Series C Financing to Accelerate Sales Expansion of The Carillon Mitral Contour System®
Funds to be Used for Commercial Expansion in Europe and Australia KIRKLAND, Washington, Sept. 29, 2020 /PRNewswire/ -- Cardiac Dimensions®, a leader in the development of innovative, minimally invasive treatments for functional mitral regurgitation (FMR) in patients with heart failure, today ann...
VolitionRx Limited Announces Virtual Capital Markets Day 2020
AUSTIN, Texas, Sept. 29, 2020 /PRNewswire/ -- VolitionRx Limited (NYSE AMERICAN: VNRX) ("Volition") a multi-national epigenetics company developing simple, easy to use, cost effective blood tests to help diagnose a range of cancers and other diseases, today announced it will host a capital market...
Guide IR Fever Warning Systems Authorized by Health Canada as Medical Device for Uses Related to COVID-19
CALGARY, AB, Sept. 29, 2020 /PRNewswire/ -- Guide Sensmart ("the Company"), a world leading manufacturer of infrared thermal imaging systems, has had its IR236 IR Fever Warning System added to the list of authorized medical devices for uses related to COVID-19 by Health Canada. The authorized dev...
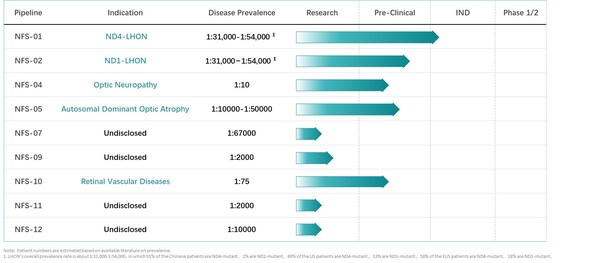
Neurophth Therapeutics' Treatment of Leber's Hereditary Optic Neuropathy Gene Therapy NR082 was Granted Orphan Drug Designation by U.S. FDA
NEWARK, Del., Sept. 24, 2020 /PRNewswire/ -- Neurophth Therapeutics, Inc., (hereinafter referred to as "Neurophth") today announced that its leading candidate, NR082 (rAAV2-ND4, NFS-01 project), was granted an orphan drug designation (ODD) by the U.S. FDA for the treatment of Leber's Hereditary O...
GenScript ProBio Congratulates XLifeSc on FDA Allowance of IND Application for TCR-T Program
NANJING, China, Sept. 24, 2020 /PRNewswire/ -- On September 24, XiangXue Life Sciences (XLifeSc), a partner of GenScript ProBio, announces FDA allowance of its IND application for TCR-T program (TCRT-ESO-A2). GenScript ProBio extends congratulations on this. This program is China's first TCR-T...
Samsung Biologics signs strategic partnership with Kanaph Therapeutics to develop treatment for retinal diseases
INCHEON, South Korea, Sept. 23, 2020 /PRNewswire/ -- Samsung Biologics (207940.KS) and Kanaph Therapeutics have entered into a strategic partnership to develop KNP-301, a bi-specific Fc fusion protein intended to treat retinal diseases. According to Kanaph Therapeutics, KNP-301 is designed to ta...
ImmVira Announces US$10 million Strategic Series B Plus Financing with SIIC Capital
SHENZHEN and SHANGHAI, Sept.23, 2020 /PRNewswire/ -- ImmVira Group Company, a biotechnology company focused on the development of new generation oncolytic viruses as potential cancer therapeutics today announced signing ofUS$10 million Series B Plus strategic financing. This round of financing wi...
Minoryx Therapeutics and Sperogenix Therapeutics enter into an exclusive license agreement to develop and commercialize leriglitazone in mainland China, Hong Kong and Macau
* Minoryx will receive an upfront and milestone payments of up to $78 million , as well as double digit royalties on annual net sales * Sperogenix will receive exclusive rights to develop and commercialize leriglitazone for the treatment of X-linked adrenoleukodystrophy (X-ALD), a rare life-th...
Alcohol Detection Anklets Showing Promise in NZ
With COVID-19, Incidents of Alcohol Abuse Reportedly on the Rise AUCKLAND, New Zealand, Sept. 22, 2020 /PRNewswire/ -- Alcohol Detection Anklets (ADA) are garnering a lot of attention in New Zealand and Australia for helping alcohol-involved clients refrain from drinking. The anklets use transde...
China Pharmaceutical Holdings Co., Ltd. Has Obtained Multiple Certifications for Its Masks
HAIKOU CITY, China, Sept. 21, 2020 /PRNewswire/ -- China Pharma Holdings, Inc. (NYSE American: CPHI) ("China Pharma," the "Company" or "We"), an NYSE American-listed corporation with a fully-integrated specialty pharmaceuticals subsidiary based inChina, today announced that the masks produced by ...
I-Mab Announces China NMPA Clearance for Phase 1 Clinical Trial of Lemzoparlimab in Relapsed or Refractory Advanced Lymphoma
SHANGHAI and GAITHERSBURG, Md., Sept. 21, 2020 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced that the Center for Drug Evaluation (CDE) of the China ...
PharmAbcine expands partnership with Samsung Biologics for PMC-403
DAEJEON, South Korea, Sept. 21, 2020 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks) entered into a strategic partnership with Samsung Biologics (KRX: 207940.KS) for the development and manufacturing of PMC-403 pipeline, the next generation therapeutic antibody candidate to treat neovascular ...
WellsCare signs in-store contract with b8ta for IASO
SEOUL, South Korea, Sept. 20, 2020 /PRNewswire/ -- WellsCare, a member company of theBorn2Global Centre, recently signed a store contract with b8ta, a famous American flagship store that is dedicated to innovative products. WellsCare has been an active member of the Born2Global Centre since 2019....
Preparation products of Yiling Pharma approved by FDA for launch in the US market
SHIJIAZHUANG, China, Sept. 19, 2020 /PRNewswire/ -- Yiling Pharmaceutical Co., Ltd. announced on Friday that it had received the approvals of the Abbreviated New Drug Applications (ANDA) about Lisinopril Tablets and Acyclovir Capsules, from United States Food and Drug Administration (hereinafter ...
MorphoSys and I-Mab Announce FDA Clearance of IND Application for MOR210/TJ210 in Patients with Advanced Cancer
PLANEGG/MUNICH, Germany and SHANGHAI, China, Sept. 17, 2020 /PRNewswire/ -- MorphoSys AG (FSE: MOR; Prime Standard Segment, MDAX & TecDAX; NASDAQ: MOR) and I-Mab (Nasdaq: IMAB) today jointly announced that the U.S. Food and Drug Administration (FDA) has cleared the Investigational New Drug applic...
Week's Top Stories
Most Reposted
LONG AN INTERNATIONAL PORT JOINS 12TH PORTECH ASIA SUMMIT 2025 IN MALAYSIA
[Picked up by 334 media titles]
2025-01-18 03:30Hong Kong Airlines Takes Off to Australia's Gold Coast Bringing Popular Travel Option for the Chinese New Year
[Picked up by 307 media titles]
2025-01-18 17:00HARD ROCK HOTEL BALI ACHIEVES PRESTIGIOUS GSTC CERTIFICATION
[Picked up by 304 media titles]
2025-01-20 14:27dss⁺ Announces Strategic Changes to Executive Leadership Team in Asia Pacific Accelerating growth and impact for high-hazard industries in the region
[Picked up by 302 media titles]
2025-01-21 16:00Infobip Research Unveils Opportunities for Brands to Engage Customers with Rich Communication Services (RCS) across APAC
[Picked up by 288 media titles]
2025-01-20 21:09