Medical/Pharmaceuticals
Minoryx Therapeutics and Sperogenix Therapeutics enter into an exclusive license agreement to develop and commercialize leriglitazone in mainland China, Hong Kong and Macau
* Minoryx will receive an upfront and milestone payments of up to $78 million , as well as double digit royalties on annual net sales * Sperogenix will receive exclusive rights to develop and commercialize leriglitazone for the treatment of X-linked adrenoleukodystrophy (X-ALD), a rare life-th...
Alcohol Detection Anklets Showing Promise in NZ
With COVID-19, Incidents of Alcohol Abuse Reportedly on the Rise AUCKLAND, New Zealand, Sept. 22, 2020 /PRNewswire/ -- Alcohol Detection Anklets (ADA) are garnering a lot of attention in New Zealand and Australia for helping alcohol-involved clients refrain from drinking. The anklets use transde...
China Pharmaceutical Holdings Co., Ltd. Has Obtained Multiple Certifications for Its Masks
HAIKOU CITY, China, Sept. 21, 2020 /PRNewswire/ -- China Pharma Holdings, Inc. (NYSE American: CPHI) ("China Pharma," the "Company" or "We"), an NYSE American-listed corporation with a fully-integrated specialty pharmaceuticals subsidiary based inChina, today announced that the masks produced by ...
PharmAbcine expands partnership with Samsung Biologics for PMC-403
DAEJEON, South Korea, Sept. 21, 2020 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks) entered into a strategic partnership with Samsung Biologics (KRX: 207940.KS) for the development and manufacturing of PMC-403 pipeline, the next generation therapeutic antibody candidate to treat neovascular ...
I-Mab Announces China NMPA Clearance for Phase 1 Clinical Trial of Lemzoparlimab in Relapsed or Refractory Advanced Lymphoma
SHANGHAI and GAITHERSBURG, Md., Sept. 21, 2020 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced that the Center for Drug Evaluation (CDE) of the China ...
WellsCare signs in-store contract with b8ta for IASO
SEOUL, South Korea, Sept. 20, 2020 /PRNewswire/ -- WellsCare, a member company of theBorn2Global Centre, recently signed a store contract with b8ta, a famous American flagship store that is dedicated to innovative products. WellsCare has been an active member of the Born2Global Centre since 2019....
Preparation products of Yiling Pharma approved by FDA for launch in the US market
SHIJIAZHUANG, China, Sept. 19, 2020 /PRNewswire/ -- Yiling Pharmaceutical Co., Ltd. announced on Friday that it had received the approvals of the Abbreviated New Drug Applications (ANDA) about Lisinopril Tablets and Acyclovir Capsules, from United States Food and Drug Administration (hereinafter ...
MorphoSys and I-Mab Announce FDA Clearance of IND Application for MOR210/TJ210 in Patients with Advanced Cancer
PLANEGG/MUNICH, Germany and SHANGHAI, China, Sept. 17, 2020 /PRNewswire/ -- MorphoSys AG (FSE: MOR; Prime Standard Segment, MDAX & TecDAX; NASDAQ: MOR) and I-Mab (Nasdaq: IMAB) today jointly announced that the U.S. Food and Drug Administration (FDA) has cleared the Investigational New Drug applic...
Dr. Steven Yu Joins ChemPartner as Vice President of Regulated Bioanalysis
SHANGHAI, Sept. 17, 2020 /PRNewswire/ -- Shanghai ChemPartner announced today the appointment ofSteven Yu, Ph.D. as Vice President of Regulated Bioanalysis at the company headquarters inShanghai, China. Dr. Yu has more than 15 years of experience in drug development and extensive expertise in...
Expanding Their Existing Strategic Collaboration, GenScript ProBio Licensed Global Rights to Develop and Commercialize a SMAB Bispecific Antibody Molecule to REMD Biotherapeutics Inc.
NANJING, China, Sept. 17, 2020 /PRNewswire/ -- On September 16th, 2020, GenScript ProBio and REMD Biotherapeutics Inc. (REMD) announced that REMD has licensed a bispecific antibody derived from the Single-domain antibody fused to Monoclonal Ab (SMAB) platform developed by GenScript ProBio. REMD i...
Samsung Biologics signs development agreement with Panolos for solid tumor treatment
INCHEON, South Korea, Sept. 16, 2020 /PRNewswire/ -- Samsung Biologics (207940.KS) has entered into a service agreement with Panolos Bioscience to develop PB101, an Fc-fusion protein intended to treat solid tumors. Under this agreement, Samsung Biologics will provide a full scope of its developm...
Transcenta Successfully Scaled up the Continuous Perfusion Process and Completed GMP Manufacturing of a Novel Bispecific Antibody for Cancer Immunotherapy
SUZHOU, China and HANGZHOU, China, Sept. 15, 2020 /PRNewswire/ -- Transcenta, a global biotherapeutics company, today announced success in scaling up the continuous perfusion process to 200L and completion of GMP production of a bispecific antibody for a Phase 1 clinical study. "This is an impo...
Verkada Introduces Environmental Sensor to Provide Enhanced Visibility into Physical Spaces
Sensors seamlessly integrate with Verkada's enterprise video security and access control solutions to deliver real-time insights into buildings Verkada accelerates business growth in Q2 2020 with 65 percent quarter-over-quarter revenue growth amidst COVID-19 pandemic SAN MATEO, California, Sept....
PharmAbcine Unveils Olinvacimab's Positive Results from Phase Ib Combination Studies at KSMO 2020
DAEJEON, South Korea, Sept. 14, 2020 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks) today announced positive data from its two combination trials of olinvacimab, its leading clinical candidate in oncology, with MSD's pembrolizumab at the 13th Annual Meeting of the Korean Society of Medical O...
Coronavirus Breakthrough: Senhwa Reports First eIND Silmitasertib Treated Severe COVID-19 Patient - Discharged Following Five Days of Treatment
TAIPEI and SAN DIEGO, Sept. 11, 2020 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a clinical-stage biopharmaceutical company focused on next generation DNA Damage Response (DDR) therapeutics for the treatment of cancer, announced today that the first patient with severe COVID-19 demonst...
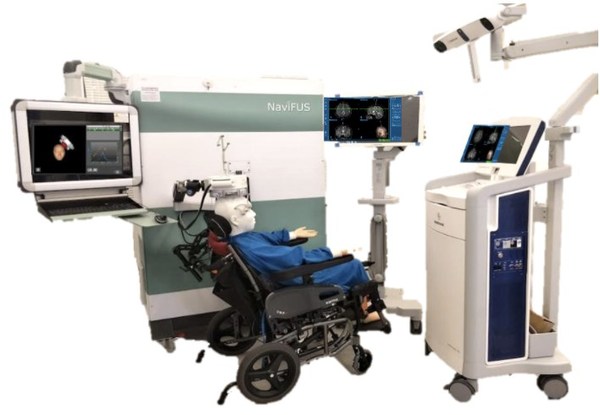
NaviFUS Launches Neuronavigation-guided Clinical Trial to Open the Blood-Brain-Barrier for Combination FUS-Bevacizumab Therapy in rGBM Patients
TAIPEI, Sept. 11, 2020 /PRNewswire/ -- Genovate Biotech (TPEX:4130) subsidiary andTaiwan-based focused ultrasound (FUS) manufacturer NaviFUS Corporation is pleased to announce the start of its clinical trial (NCT04446416) for the combination of FUS plus bevacizumab therapy. Researchers at Linkou ...
Tottenham Acquisition I Limited Announces Filing of a Registration Statement on Form S-4 in Connection with its Proposed Business Combination with Clene Nanomedicine, Inc.
NEW YORK, Sept. 10, 2020 /PRNewswire/ -- Tottenham Acquisition I Limited (Nasdaq: TOTA, TOTAU, TOTAW, TOTAR) ("Tottenham"), a publicly traded special purpose acquisition company, announced today that its subsidiary, Chelsea Worldwide Inc., has filed with the U.S. Securities and Exchange Commissio...
CMAB Biopharma and Laekna Therapeutics Enter Strategic Agreement for LAE005 Global Development and Commercialization Partnership
SUZHOU, China, Sept. 10, 2020 /PRNewswire/ -- CMAB Biopharma (Suzhou) Inc. ("CMAB"), and Laekna Therapeutics Shanghai Co., Ltd. ("Laekna Therapeutics"), today announced a strategic collaboration agreement in Suzhou BioBAY for speedup of Immune Checkpoint Inhibitor (ICI) drug candidate to the clin...
The Global Clinical CRO Fountain Medical Development and its Affiliates Rebrand as ClinChoice Inc
BEIJING and SHANGHAI, Sept. 9, 2020 /PRNewswire/ -- Fountain Medical Development (FMD K&L), together with its affiliates, is rebranding as ClinChoice Inc ("ClinChoice" or "Company"). ClinChoice is a global clinical stage Contract Research Organization ("CRO"), with over 1800 clinical research ...
Clarity Pharmaceuticals Announces the US FDA Grants Rare Paediatric Disease Designation to 64Cu-SARTATE™, a diagnostic for the clinical management of neuroblastoma
SYDNEY, Sept. 9, 2020 /PRNewswire/ -- Clarity Pharmaceuticals, a clinical-stage radiopharmaceutical company focused on the treatment of serious disease, is pleased to announce that the U.S. Food and Drug Administration (FDA) has granted Rare Paediatric Disease Designation (RPDD) to64Cu-SARTATE™, ...
Week's Top Stories
Most Reposted
ASTON MARTIN ARAMCO FORMULA ONE® TEAM ANNOUNCE PEPPERSTONE AS OFFICAL TRADING PARTNER
[Picked up by 307 media titles]
2025-01-07 09:00Brewing Connections and Spotlighting Café Culture: MIFB 2025 Hosts MNCC in Collaboration with MSCA
[Picked up by 305 media titles]
2025-01-06 14:00RuggON Unveils VORTEX Vehicle Mount Computer with AI-Enhanced, SATCOM-Ready
[Picked up by 305 media titles]
2025-01-06 21:00iWOW Unveils Age-Tech Innovations at CES 2025, Expanding Portfolio of IoT Solutions for Ageing-In-Place
[Picked up by 290 media titles]
2025-01-07 21:18CELEBRATE THE LUNAR NEW YEAR 2025 AT HOIANA RESORT & GOLF, WORLD'S LEADING FULLY INTEGRATED RESORT
[Picked up by 289 media titles]
2025-01-06 19:25